A perfluorohexyl containing diketopyrrolopyrrole (DPP) small molecule for high performance ambipolar transistors with balanced hole and electron mobilites
Abstract
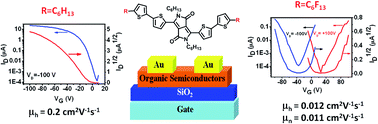
Two novel diketopyrrolopyrrole (DPP) small molecules DPPTT-F and DPPTT-H were synthesized. With the change of substituent from hexyl to perfluorohexyl, the HOMO and LUMO energy levels of DPPTT-F lowered, but the bandgap of DPPTT-H and DPPTT-F was nearly the same, which was proved by absorption spectra and electrochemistry. Thin film transistor characteristics showed DPPTT-H exhibited p-channel behavior with a hole mobility of about 0.2 cm2 V−1 s−1, while DPPTT-F displayed ambipolar characteristics with balanced hole and electron mobility in air. The mobility of DPPTT-F was 0.012 cm2 V−1 s−1 for holes and 0.011 cm2 V−1 s−1 for electrons. The microstructure and morphology of the thin films were also investigated. All these results demonstrated the unique property of perfluoroalkyl chains and their potential application in high performance organic ambipolar semiconductors.

 Please wait while we load your content...
Please wait while we load your content...