New understanding in the influence of melt structure and β-nucleating agents on the polymorphic behavior of isotactic polypropylene
Jian Kanga,
Gengsheng Wengb,
Zhengfang Chena,
Jinyao Chena,
Ya Caoa,
Feng Yang*a and
Ming Xianga
aState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, People's Republic of China. E-mail: yangfengscu@126.com
bFaculty of Materials and Chemical Engineering, Ningbo University, Ningbo 315211, People's Republic of China
First published on 16th June 2014
Abstract
The β-nucleation behavior of isotactic polypropylene (iPP) is a fascinating and important issue in polymer physics; however, little about this phenomenon or its physical nature has been clearly understood. In the present study, by tuning the heating temperature (fusion temperature, Tf), the amount of ordered structures in iPP melt was controlled. In this way, the influence of five types of representative β-nucleating agents (β-NA) on the crystallization behavior of iPP with different melt structures (i.e. the amount of ordered structures) was comparatively studied by differential scanning calorimetry (DSC), polarized optical microscopy (PLOM), scanning electron microscopy (SEM), wide-angle X-ray diffraction (WAXD) and rheological measurement. A surprising synergetic effect was observed between β-NAs with α-/β-dual polymorphic selectivity (dual-selective β-NA) and the ordered structure of iPP, resulting in significant increases of the β-nucleation efficiency and the β-phase proportion of the sample. PLOM observation of the crystallization process confirmed that with the presence of ordered structures in iPP melt, a mass of dark, small crystal embryos derived from self-nuclei uniformly distributed in the melt and exhibited β-nucleation efficiency. This work provides the first evidence that for iPP nucleated with dual-selective β-NA, the ordered structures play a determining role in the β-nucleation of iPP. Under the influence of the dual-selective β-NA, the ordered structures exhibited β-nucleation efficiency and therefore encouraged β-nucleation. A possible mechanism was proposed.
1. Introduction
Polymer crystallization is a long-standing challenge in polymer physics, attracting broad interest from scientific and industrial aspects owing to its great importance. During the last 60 years, great attention has been paid and many different theories or models have been developed,1,2 however no full agreement was reached.In the early stage of polymer crystallization, whether the ordered structure exists in the melt before the occurrence of the real crystallization or not is an important issue under debate.1–8 A widely accepted viewpoint proposes that polymer chains in the melt are in a random coil state at high temperature, which will not form any ordered structures even at the level approaching the diameter of the chains. However, more and more experimental results indicate that some ordered structures may exist in supercooled polymer melt before the occurrence of real crystallization.9–14 In the induction period, a spinodal-assisted crystallization mechanism has been proposed mainly based on the scattering and spectral results; at the growth front, Strobl G.1 proposed a multistage process model, where a transient mesomorphic layer is proposed at the growth front of crystal. Li L. B.15,16 studied the role of ordered structures in the growth front of isotactic polypropylene (iPP) and observed a growth front layer with ordered long helices outside the growth front of spherulite. The results showed the presence of preordering on the growth front of iPP spherulite.
It is reported that the locally ordered structures can also be created by other methods such as self-nucleation.17–21 When the heating temperature (fusion temperature, Tf) is not high enough to completely melt the polymer, an amount of ordered structures will survive within the melt. Therefore, the content of locally ordered structures can be tuned by controlling the Tf applied on the polymer. Although the physical nature of the ordered structures is still under debate,22 it is commonly accepted that they can serve as self-nucleating agents during the crystallization process, enhancing the nucleation density and crystallization rate.17,22,23
As a typical semi-crystalline polymer and one of the most widely used commercial polymers, iPP exhibits pronounced polymorphisms and morphologies,24–29 which can crystallize into several crystal modifications known as monoclinic α-form,30 trigonal β-form31–33 and orthorhombic γ-form.34 The β-form is a metastable crystalline phase and can be obtained only under several specific conditions such as shearing,35 using specific nucleating agents36–44 and directional crystallization in a thermal gradient.45 Although the role of ordered structures in the α-phase crystallization of iPP has been well recognized, the relationship between the ordered structure and the β-crystallization has rarely been reported.
Recently, by introducing partially melting iPP fiber to a homogeneous supercooled iPP melt, Yan S. K. et al.46–50 systematically investigated the β-crystallization of iPP/iPP fiber single composite and claimed that the partial melting of orientated fibers may provide the locally ordered structures within certain orientation windows, which play a very important role in the β-crystallization; Alfonso G. C. et al.51–53 studied the formation and relaxation of shear-induced nucleation and found that shear flow and partially molten fibers have similar effects on the formation of β-phase; Shen C. Y. et al.54,55 explored the combined effect of ordered structures and shear flow on the polymorphic nature of β-cylindrites and found that only in the presence of ordered structures can iPP melt form β-cylindrites under the influence of shear flow, showing that the ordered structures under shearing are crucial to the β-crystallization of iPP. Although the crystallization conditions and components of the above studies are quite different from each other, it should be emphasized that the two-stage characteristics of the β-nucleation in these studies are, to some extent, very similar: (i) at the early stage of crystallization, the row nucleated α-lamellae form first along the shear stress/fiber direction. (ii) Then the β-nucleation occurs on the edge of the row nucleated α-lamellae, and a competitive growth of both α- and β-crystal occurs.
The above-mentioned studies make breakthroughs in relating the ordered structures to the β-crystallization of iPP and provide a possibility that by applying certain orientation states, these ordered structures may exhibit β-nucleation efficiency and therefore encourage the β-crystallization. However, to the best of our knowledge, it seems that in the quiescent iPP melt without shearing or orientated fibers, the role of the ordered structures in the β-nucleation of iPP is still not recognized; neither the phenomenon nor the general nature of the β-crystallization of iPP in different crystallization conditions (i.e., shearing, thermal gradient and β-nucleating agents) is clearly understood, which still needs further investigation.
The addition of β-nucleating agent (β-NA) is a commonly used method to enhance β-crystallization of iPP. Due to the limitations of the characterization techniques and the distinct characteristics of various β-NAs such as chemical constitution, polymorphic selectivity, β-nucleation efficiency and geometric shape,36,38 the β-nucleation mechanism of the β-NAs is still not well understood although some important theories such as the epitaxial crystallization mechanism have been proposed.56 In contrast, various NAs with distinct characteristics provide us an opportunity to tune their influence on the ordered structures of iPP, namely, a possibility to tune the β-nucleation efficiency of the ordered structures.
By investigating the β-crystallization behavior of the nucleated iPP with different melt structures (i.e. different contents of ordered structures), this study aims to explore the influence of various NAs on the ordered structures in iPP melt, so as to elucidate the novel understanding of the role of ordered structures in β-nucleation of quiescent iPP melt, as well as the induction mechanism of β-NA in the β-crystallization of iPP.
2. Experimental section
2.1 Materials
iPP, tradename T38F (Lanzhou OilChem Corp., China) with average isotacticity 97.6%, weight molecular weight 347200, and polydispersity index = 3.63 was used.The five nucleating agents (NAs) used in this study are commercially available products, which are widely used and extensively studied in previous studies. The detailed information of the NAs including tradename, manufacturer, chemical composition, polymorphic selectivity are listed in Table 1. Moreover, their geometric shapes are observed by SEM as shown in Fig. 1.
As can be seen from Fig. 1 and Table 1, the five NAs can be classified into different groups: according to the polymorphic selectivity, DMDBS is a highly efficient α-NA,39 meanwhile, Pa–Ca and NAB83 are β-NAs with β-single polymorphic selectivity,44,57 DCNDCA and WBG-II are β-NAs with α-/β-dual polymorphic selectivity (denoted as dual-selective β-NA in this study);58–61 from the point of view of geometric shape, DMDBS and DCNDCA are club-shaped, Pa–Ca is a flaky staff, NAB83 is a rod-like entity, and WBG-II is an irregular block-like crystal. Meanwhile, the sizes of these NAs are also different from each other.
2.2 Sample preparation
The iPP pellets and NA were mixed in a weight ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then extruded by a twin-screw extruder (SHJ-20, Nanjing Giant Machinery Co., Ltd, China) and pelletized to obtain a master batch. The master batch and iPP were mixed and extruded by twin-screw again to obtain β-iPP. The concentration of each NA was 0.03 wt%.
1 and then extruded by a twin-screw extruder (SHJ-20, Nanjing Giant Machinery Co., Ltd, China) and pelletized to obtain a master batch. The master batch and iPP were mixed and extruded by twin-screw again to obtain β-iPP. The concentration of each NA was 0.03 wt%.
2.3 Differential scanning calorimetry (DSC)
All the calorimetric experiments were performed with a Mettler Toledo DSC1 (Mettler Corp., Swiss) differential scanning calorimeter (DSC) under nitrogen atmosphere (50 mL min−1). The temperature scale calibration was performed using indium as a standard to ensure reliability of the data obtained. In addition, 5 mg round samples were used. All the thermograms were fitted using Peakfit 4.12 software according to literature.62,63 In this way, the single melting curve of the β-iPP was separated into individual peaks; therefore, the relative percentage of β-phase was calculated. The relative percentage crystallinities of α-crystal (αc) and β-crystal (βc) were estimated by the following expressions:| αc = Xα/(Xβ + Xα) | (1) |
| βc = Xβ/(Xβ + Xα) | (2) |
In DSC measurement, the thermal treatment described in Scheme 1 is applied to create iPP with different melt structures (namely, to tune the presence and amount of ordered structures in the iPP melt). The sample is first heated to 200 °C and held for 5 min to erase any previous thermal history. Then it is cooled to 50 °C at 10 °C min−1 to create a “standard” thermal history. Further, it is heated to different fusion temperatures (Tf, ranging from 167–200 °C) at 10 °C min−1 and held for 5 min to create different melt structures, namely, to control the presence and content of the ordered structures in the melt. Then, it is cooled down to 50 °C at 10 °C min−1. Finally, it is heated to 200 °C at 10 °C min−1.
2.4 Wide-angle X-ray diffraction (WAXD)
WAXD patterns were recorded with a DX-1000 diffractometer. The wavelength of CuKα was λ = 0.154 nm, and the spectra were recorded in the 2θ range of 5–35°, a scanning rate of 2° min−1, and a scanning step of 0.02°.The content of the β-crystal was determined according to standard procedures described in the literature,40,64,65 employing the following equation:36
 | (3) |
2.5 Polarized optical microscopy (PLOM)
The morphology evolution during crystallization was studied with a ZEISS MC-80 polarized light microscope equipped with a LINKAMTP-91 hot-stage and a camera system (ZEISS Co. Ltd., Germany). To enhance contrast, a λ wave plate was inserted between the polarizers.2.6 Scanning electron microscopy (SEM)
The morphology observation (Scanning Electronic Microscopy, SEM) was performed on a JSM-5900 LV environmental scanning electron microscope at an accelerating voltage of 20 kV. Before SEM characterizations, the surfaces of all the samples were coated with a thin layer of gold by ion sputtering. All the samples were etched for 2 h in a solution containing 1.3 wt% potassium permanganate (KMnO4), 32.9 wt% concentrated sulfuric acid (H2SO4) and 65.8 wt% concentrated phosphoric acid (H3PO4), according to the procedure proposed by Olley and Bassett.662.7 Rheological measurement
The rheological measurements were performed with a stress-controlled Gemini 200 rheometer (Malvern Instruments Ltd, UK) in the linear viscoelastic regime under a nitrogen atmosphere, and the selected stress was 10 Pa. Testing sample disks with a diameter of 25 mm and a thickness of 1.5 mm were prepared by compression molding of the PP pellets at 190 °C for 5 min. Each specimen was initially heated to 220 °C and held for 5 min. A dynamic temperature sweep mode was applied at an oscillation frequency of 0.1 rad s−1, and the temperature was subsequently decreased at a rate of 3 °C min−1 until the solidification of iPP occurred.3. Results and discussion
3.1 Preliminary analysis of the crystallization behavior
The influence of the nucleating agents (NAs) on the crystallization and melting behavior of iPP is studied using DSC and WAXD. Fig. 2 shows the DSC cooling curves and subsequent heating curves, and the WAXD profiles of the samples with the same thermal history. The crystallization temperature (Tc) on DSC cooling curves, the relative percentage of β-phase calculated from DSC heating curves (βc) and WAXD profiles are shown in Fig. 3a–c, respectively. Note that multiple peaks emerge in the DSC heating profiles of some samples. According to the literature36,67 and the WAXD results in Fig. 2c, the melting peaks in the temperature range lower than 155 °C are classified as β-phase melting peaks, while the melting peaks that emerge above 155 °C are the α-phase melting peaks. | ||
| Fig. 2 (a) Cooling curves, (b) subsequent heating curves of the pure iPP and the nucleated iPP samples and (c) the WAXD profiles of the samples with the sample thermal history. | ||
 | ||
| Fig. 3 (a) The crystallization peak temperature, (b) relative percentage of β-phase (βc) calculated from DSC heating curves and (c) the β-phase proportion (kβ) calculated from the WAXD profiles | ||
On the cooling curves, the Tc increases after the addition of each NA due to the nucleating effects. Meanwhile, on the DSC heating curves and WAXD profiles (Fig. 2b and c), the polymorphic behavior of the nucleated iPP is greatly dependent on the characteristics of the NAs: β-crystallization cannot occur in the DMDBS-nucleated iPP due to its α-single polymorphic selectivity; iPP nucleated with Pa–Ca and NAB83 show high β-phase proportion (βc from DSC and kβ from WAXD), reflecting the high β-nucleation efficiency of the NAs; iPP nucleated with DCNDCA and WBG-II only form a relatively low proportion of β-phase since DCNDCA and WBG-II are dual-selective β-NAs having lower β-nucleation efficiency compared with NAB83 and Pa–Ca.
3.2 Crystallization behavior of nucleated iPP with different melt structures
By tuning the fusion temperature (Tf) using the thermal treatment protocol described in Scheme 1, the content of ordered structures in iPP melt is efficiently controlled. In this section, the crystallization behaviors of iPP samples with different contents of ordered structures are studied. | ||
| Fig. 4 Cooling curves of the samples after held at different fusion temperatures Tf for 5 min. The concentrations of the NAs are 0.03 wt%, and the cooling rate is 10 °C min−1. | ||
As can be seen from Fig. 4 and 5, for pure PP, when the Tf is higher than 171 °C, the crystallization temperature Tc stays almost constant, reflecting the constant nucleation density of the melt, namely, iPP is fully molten. In the Tf range of 171–168 °C, Tc gradually increases with the decrease of Tf, indicating the presence of the ordered structures in the melt and the occurrence of self-nucleation; as Tf decreases to less than 168 °C, the crystallization immediately happens during the subsequent cooling due to the presence of large ordered entities that can anneal or recrystallize during the cooling.
When the NA is added, for DMDBS, NAB83 and Pa–Ca, the crystallization peak temperature Tc stays almost unchanged with the decrease of Tf, indicating that for iPP nucleated with these NAs, the Tc is independent of Tf; however, for DCNDCA and WBG-II, an evident dependence of Tc on Tf is observed. When Tf is higher than 189 °C, Tc remains unchanged due to the constant nucleation density in the melt; when Tf is in the temperature range of 168–189 °C, the Tc increases gradually with the decrease of Tf due to the increase of the amount of ordered structures within the melt;18,20,21 when Tf decreases to lower than 168 °C, the crystallization peak significantly widens and two peaks can be observed, indicating that Tf is so low that the large ordered entities can survive in the melt,20 which are large enough to induce annealing or recrystallization in the course of subsequent cooling and therefore induce a second crystallization peak in the high temperature range.
 | ||
| Fig. 6 Heating curves (Curve 2 in Scheme 1) of (a) the pure iPP and iPP nucleated with (b) DMDBS, (c) Pa–Ca, (d) NAB83, (e) DCNDCA and (f) WBG-II after being held at the indicated Tf and then cooled to 50 °C. The cooling and heating rates are 10 °C min−1. | ||
 | ||
| Fig. 7 Plots of the relative contents of β-phase βc of iPP nucleated with (a) DCNDCA and (b) WBG-II as a function of the fusion temperature Tf. The error bars and statistics are also provided. | ||
Fig. 6a shows that for pure iPP, the melting profile stays constant when the Tf is higher than 167 °C. When the Tf decreases to 167 °C or less, a small melting peak is observed at the high melting temperature range, corresponding to the melting of the thick lamellaes due to the annealing and recrystallization of the surviving large ordered entities at high temperature.20,21 The crystallization and melting behavior of pure iPP is in good agreement with the conventional self-nucleation behavior of iPP in previous studies.
On the other hand, the melting profiles of iPP nucleated with DMDBS, Pa–Ca and NAB83 (Fig. 6b–d) stay almost unchanged when Tf varies in the temperature range of 170–200 °C. As the Tf decreases to 165 °C, the β-melting peak located at 140–155 °C disappears attributed to the depression effect of the surviving large ordered entities on β-crystallization. The β-crystallization behaviors of iPP nucleated with DMDBS, Pa–Ca and NAB83 are in accordance with those reported in previous studies, showing that in these cases the used NAs cannot trigger any β-nucleation efficiency of the ordered structure.
For iPP nucleated with DCNDCA/WBG-II (Fig. 6e and f), when Tf is higher than 189 °C, the sample is completely melted, and the relative percentages of β-phase (βc) on the heating curve are relatively low; surprisingly, when Tf is in the temperature range of 168–189 °C, the β-melting peak becomes evidently larger. Meanwhile, the βc significantly increases for iPP nucleated with DCNDCA and WBG-II, from 18.2% and 36.6% (when Tf is higher than 189 °C) to 84.1% and 80.3% at maximum, respectively. When Tf is not higher than 167 °C, βc sharply decreases due to the α-nucleation effect from the surviving large ordered entities as mentioned above. The surprising results above indicate that the polymorphic behavior of iPP nucleated with DCNDCA/WBG-II is greatly dependent on the fusion temperature Tf, in other words, the presence of ordered structures in the melt. There exists a synergetic effect between the specific β-NA of DCNDCA/WBG-II and the ordered structures of iPP, which can evidently increase the β-nucleation efficiency of the sample.
According to the variation of crystallization peak temperature Tc on the cooling curves and the relative percentage of β-phase (βc) on the DSC heating curves with the change of fusion temperature (Tf), the whole Tf temperature range can be divided in to three regions as indicated by the dotted lines in Fig. 7:
(i) Region I (Tf > 189 °C in this study), where iPP is fully molten, the nucleation density in the melt is constant, Tc and βc are constant;
(ii) Region II (168 °C ≤ Tf ≤ 189 °C), where iPP is partially melted, and an amount of ordered structures survive in the melt. Due to the synergetic effect between the specific β-NA and the ordered structures of iPP, the β-nucleation efficiency of the sample is evidently elevated, resulting in the increase of Tc and βc;
(iii) Region III (Tf < 168 °C), where the large ordered entities that can induce annealing or recrystallization during the subsequent cooling can survive in the melt, resulting in the emergence of the second crystallization peak and the sharp decrease of βc.
To benefit discussion, the synergetic effect between the specific β-NA and the ordered structures of iPP when the Tf is in Region II is named the Ordered Structure Effect (OSE).
3.3 Effect of the supercooled treatment and holding time at fusion temperature
In order to obtain more understanding of the OSE, iPP nucleated with 0.03 wt%, WBG-II is selected as a representative sample, and two comparative studies are performed according to the thermal treatment protocols shown in Fig. 9a and b. In Thermal Treatment 2 [Fig. 9a], the sample is first heated to 200 °C and held for 5 min to erase any previous history. It is then cooled to the indicated temperature (ranging from 168–200 °C) and held for 5 min there, in order to create the iPP melt with different supercooled degrees. Further, it is cooled to 50 °C and subsequently heated to 200 °C. All the cooling and heating rates are 10 °C min−1. The obtained results [Fig. 9c–d] show that the cooling and heating curves of the sample are not influenced by the supercooled degree and are similar to those under the thermal treatment of direct cooling from 200 °C. Namely, without the presence of ordered structures in the melt, the sample nucleated with WBG-II cannot exhibit extra β-phase selectivity when the supercooled degree varies. The evident increase of the β-phase proportion in Fig. 6–8 is attributed to the synergetic effect between the ordered structures of iPP and the specific β-NA of DCNDCA and WBG-II.Previous studies have reported that the ordered structures surviving in the melt are unstable and will gradually disappear when the holding time at the fusion temperature is long enough.22 In order to study the thermal stability of the ordered structures under the influence of the specific β-NAs, the Thermal Treatment 3 described in Fig. 9b was applied. As the fusion temperature Tf ranges from 168–186 °C [in Fig. 9b, Tf is 180 °C as a representative], the results (Fig. 9e and f) elucidate that even holding at Tf in Region II for 120 min, the obtained cooling and heating curves remain almost unchanged, indicating that under the influence of the specific β-NAs, the ordered structures have a high thermal stability and can survive in the melt for a long time, which is quite different from ordered structures in pure iPP melt reported previously21,22 due to the presence of the specific β-NA [0.03 wt% WBG-II].
3.4 Morphology and structure evolution during crystallization
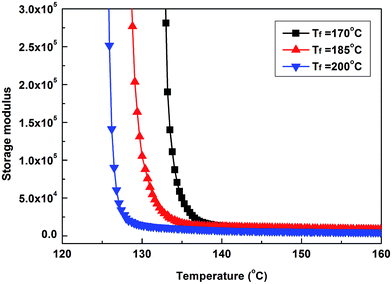 | ||
| Fig. 10 Storage modulus as a function of temperature of iPP nucleated with WBG-II cooled from 200 °C at the rate of 3 °C min−1. | ||
As the fusion temperature Tf varies from 200 °C to 185 and 170 °C, it can be seen that the inflection point of the curve gradually shift towards a higher temperature, suggesting that the crystallization occurs at higher temperatures due to the increase of the amount of ordered structures.
Fig. 11 reveals that when Tf = 200 °C, two kinds of crystal embryos can be observed at the early stage of the crystallization: one is the bright, large crystal nucleated by the β-NA, the other is the small, dark crystal mainly derived from density fluctuation.20,22,67 As the crystallization proceeds, these two kinds of crystal embryos gradually grow and form large-sized β- and α-crystals, respectively. Finally, a mixture of α- and β-phase with low β-phase content is obtained. When Tf is 185 °C, the density and location of crystal embryos nucleated by WBG-II is quite similar with the case of Tf = 200 °C. Meanwhile, more small crystal embryos can be observed. Surprisingly, a large part of these small, dark crystal embryos exhibit β-nucleation efficiency, resulting in a mass of β-crystals as the crystallization proceeds. Finally, more β-phase with smaller sizes is obtained; when Tf is 170 °C, a mass of small, uniformly distributed crystal embryos can be observed at the beginning of the crystallization. Moreover, after crystallization, large amounts of small-sized bright β-crystals almost fill up the screen, indicating the formation of a high proportion of β-phase. It should be noted that these small, dark crystal embryos seem to be uniformly distributed in the melt and are not intensively concentrated around the bright, large crystal embryos induced by the WBG-II, reflecting the typical characteristics of self-nucleation.
On the other hand, the additional experimental results of PLOM observation of the crystallization procedure of pure iPP under the same thermal treatment showed only the elevated crystallization temperature, the increase of α-nucleation sites and the decrease of α-crystallite sizes, which is in accordance with the classical self-nucleation behavior reported in the literature.18,22
The PLOM results above indicate that when Tf is in Region II (170, 185 °C), the β-phase proportion is enhanced because the ordered structures surviving in the partial melt have high β-nucleation efficiency. This is the first report of the presence of ordered structures with high β-selectivity in the quiescent iPP melt. Although the physical nature of this phenomenon is still not clear, a basic principle of this phenomenon is evidently observed: in the absence of the specific β-NA, the ordered structures within the quiescent partial melt have no β-nucleation efficiency and cannot induce β-crystallization. With the help of the specific β-NA, the ordered structures begin to possess β-nucleation efficiency, i.e., the presence of specific β-NA plays a key role in this process. In general, a synergetic effect takes place between the specific β-NA and the ordered structures of iPP, resulting in the high β-nucleation efficiency of the ordered structures and therefore significantly enhances the β-crystallization of the sample.
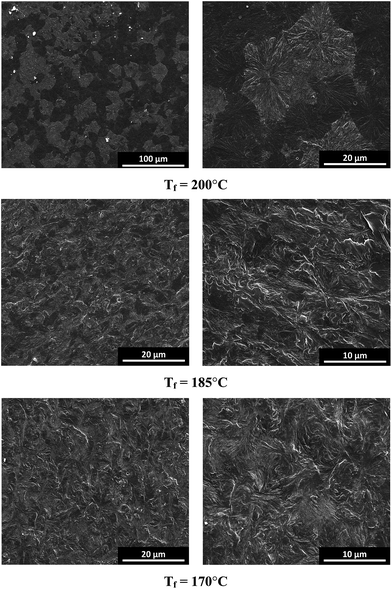 | ||
| Fig. 12 SEM images (after etching) of the iPP nucleated with 0.03 wt% WBG-II after holding at the indicated fusion temperature (Tf) for 5 min and then cooling to room temperature. | ||
When Tf = 200 °C, large spherulites with diameter of about 20 μm can be observed; Meanwhile, the β-phase (bright spherulites in the SEM image)69–71 content is relatively low. As Tf comes to 185 and 170 °C, the spherulite sizes decrease sharply, and the β-phase content increases significantly. It should be noted that in all SEM images, only the β-spherulites can be observed.
3.5 Discussion
 | ||
| Fig. 13 Schematic illustration of the two-stage β-nucleation mechanism of β-iPP nucleated with a dual-selective β-nucleating agent. | ||
Fig. 13a shows that for fully melting β-iPP (namely, when the fusion temperature Tf is in Region I), the β-crystallization may take place in a two-step manner: in Step 1, under the influence of the dual-selective β-NA, the disordered iPP chains arrange into locally ordered structures, which have high β-nucleation efficiency; in Step 2, the ordered structures having high β-nucleation efficiency encourage the occurrence of β-crystallization.
In Fig. 13b, by tuning the Tf, the content of ordered structures in the iPP melt is intentionally controlled. In this way, the amount of ordered structures in iPP during the early stage of crystallization is significantly elevated. Under the influence of the dual-selective β-NA, the ordered structures may exhibit β-nucleation efficiency, therefore, the β-nucleation efficiency of the sample is significantly enhanced, resulting in the significant increase of β-phase proportion.
Interestingly, Varga J.,58 Yu J.,61,72 Qiang F. et al.59,60 investigated the β-crystallization behavior of iPP nucleated with several types of dual-selective β-NAs, showing that these β-NAs have many similarities such as solubility in the iPP matrix and self-assembly behavior; they can encourage both α- and β-nucleation in a similar manner. Moreover, Varga J.58 found that the lateral surface of DCNDCA leads to a two-stage β-nucleation, which is surprisingly similar to that induced by the ordered structures in certain orientation windows: at the early stage of crystallization, the row of nucleated α-lamellae first forms along the side surface of the NA. Furthermore, the β-nucleation takes place on the edge of the row of nucleated α-lamellae and then a competitive growth of both α- and β-crystal occurs.
Based on the studies concerning the β-crystallization induced by orientated locally ordered structures and the dual-selective β-NAs, a possible explanation of the interaction between ordered structures and the dual-selective β-NAs is that the presence of dual-selective β-NAs may enable the ordered structures to possess certain orientation states and therefore exhibit high β-nucleation efficiency. In other words, the β-nucleation of iPP in cases such as crystallization under shear stress/oriented iPP homogeneous fibers and the addition of dual-selective β-NA may have the same nature. Meanwhile, the ordered structure in certain orientation status is the crucial factor in all of these cases.
On the other hand, since the concentration of the dual-selective β-NA is directly related to the interaction between iPP chains and the β-NA, it may be a key factor in the occurrence of the Ordered Structure Effect (OSE), which can explain why previous studies with the addition of high β-NA concentrations cannot induce the OSE observed in this study. The role of β-NA concentration in the OSE of iPP should be further investigated.
4. Conclusions
In this study, we selected five types of representative nucleating agents (NA) and investigated their influence on the crystallization and polymorphic behavior of iPP with different melt structures (i.e., the amount of ordered structures) using DSC, WAXD, PLOM, SEM and rheological measurement. It was found that a synergetic effect between the ordered structures of iPP and the β-NA with α-/β-dual selectivity (dual-selective β-NA) takes place, resulting in an evident enhancement of the β-nucleation efficiency of the samples and great increase of the β-phase proportion, which is called the Ordered Structure Effect (OSE). The ordered structures in iPP melt play a determining role in the β-nucleation process of iPP nucleated with dual-selective β-NAs, meanwhile, the dual-selective β-NA provides a certain influence on the ordered structures, thereby showing a high β-nucleation efficiency. A possible mechanism was proposed.Acknowledgements
We express our sincere thanks to the Sichuan University Scientific Research Foundation for Young Teachers (2012SCU11075) and National Science Foundation of China (NSFC 51203106) for the financial support.References
- G. Strobl, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 3, 165–183 CrossRef CAS.
- J. J. I. Lauritzen and J. D. Hoffman, J. Appl. Phys., 1973, 44, 4340–4352 CrossRef CAS PubMed.
- B. Lotz, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 3, 185–194 CrossRef CAS.
- M. Muthukumar, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 3, 199–202 CrossRef CAS.
- L. Li and W. de Jeu, in Interphases and Mesophases in Polymer Crystallization II, Springer Berlin Heidelberg, 2005, vol. 181, ch. 107175, pp. 75–120 Search PubMed.
- S. Z. D. Cheng, C. Y. Li and L. Zhu, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 3, 195–197 CrossRef CAS.
- G. Strobl, Prog. Polym. Sci., 2006, 31, 398–442 CrossRef CAS PubMed.
- J. D. Hoffman and R. L. Miller, Polymer, 1997, 38, 3151–3212 CrossRef CAS.
- R. H. Gee, N. Lacevic and L. E. Fried, Nat. Mater., 2006, 5, 39–43 CrossRef CAS PubMed.
- A. Sanz, A. Nogales, I. Puente-Orench, M. Jimenez-Ruiz and T. A. Ezquerra, Phys. Rev. Lett., 2011, 107, 025502 CrossRef.
- M. Soccio, A. Nogales, N. Lotti, A. Munari and T. Ezquerra, Phys. Rev. Lett., 2007, 98, 037801 CrossRef CAS.
- T. K. G. Matsuba, M. Saito, K. Kaji and K. Nishida, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 1–4 CrossRef.
- C. L. Nitash and P. Balsara, Phys. Rev. Lett., 1996, 77, 3847–3850 CrossRef.
- K. Kaji, K. Nishida, T. Kanaya, G. Matsuba, T. Konishi and M. Imai, in Interphases and Mesophases in Polymer Crystallization III, ed. G. Allegra, Springer Berlin Heidelberg, 2005, 191, 13, 187–240 Search PubMed.
- Y. Cong, Z. Hong, W. Zhou, W. Chen, F. Su, H. Li, X. Li, K. Yang, X. Yu, Z. Qi and L. Li, Macromolecules, 2012, 45, 8674–8680 CrossRef CAS.
- Y. Cong, Z. Hong, Z. Qi, W. Zhou, H. Li, H. Liu, W. Chen, X. Wang and L. Li, Macromolecules, 2010, 43, 9859–9864 CrossRef CAS.
- X. Li, F. Su, Y. Ji, N. Tian, J. Lu, Z. Wang, Z. Qi and L. Li, Soft Matter, 2013, 9, 8579–8588 RSC.
- B. Fillon, J. Wittmann, B. Lotz and A. Thierry, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1383–1393 CrossRef CAS.
- D. Cavallo, L. Gardella, G. Portale, A. J. Muller and G. C. Alfonso, Polymer, 2014, 55, 137–142 CrossRef CAS PubMed.
- A. J. Muller and M. L. Arnal, Prog. Polym. Sci., 2005, 30, 559–603 CrossRef PubMed.
- A. T. Lorenzo and A. J. Müller, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1478–1487 CrossRef CAS.
- A. T. Lorenzo, M. L. Arnal, J. J. Sánchez and A. J. Muller, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1738–1750 CrossRef CAS.
- B. Fillon, B. Lotz, A. Thierry and J. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1395–1405 CrossRef CAS.
- J. Kang, B. Xiong, D. Liu, Y. Cao, J. Chen, F. Yang and M. Xiang, J. Polym. Res., 2014, 21(485), 1–10 Search PubMed.
- J. Kang, J. Li, S. Chen, S. Zhu, H. Li, Y. Cao, F. Yang and M. Xiang, J. Appl. Polym. Sci., 2013, 130, 25–38 CrossRef CAS.
- J. Kang, F. Yang, T. Wu, H. Li, D. Liu, Y. Cao and M. Xiang, J. Appl. Polym. Sci., 2012, 125, 3076–3083 CrossRef CAS.
- J. Kang, F. Yang, T. Wu, H. Li, Y. Cao and M. Xiang, Eur. Polym. J., 2012, 48, 425–434 CrossRef CAS PubMed.
- J. Kang, Y. Cao, H. Li, J. Li, S. Chen, F. Yang and M. Xiang, J. Polym. Res., 2012, 19(37), 1–11 CAS.
- D. Liu, J. Kang, M. Xiang and Y. Cao, J. Polym. Res., 2013, 20(126), 1–10 CrossRef CAS PubMed.
- G. Natta and P. Corradini, Nuovo Cimento, 1960, 15, 40–51 CrossRef CAS.
- D. L. Dorset, M. P. McCourt, S. Kopp, M. Schumacher, T. Okihara and B. Lotz, Polymer, 1998, 39, 6331–6337 CrossRef CAS.
- B. Fillon, A. Thierry, J. Wittmann and B. Lotz, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1407–1424 CrossRef CAS.
- D. R. Ferro, S. V. Meille and S. Bruckner, Macromolecules, 1998, 31, 6926–6934 CrossRef CAS.
- S. Bruckner, P. J. Phillips, K. Mezghani and S. V. Meille, Macromol. Rapid Commun., 1997, 18, 1–7 CrossRef.
- A. Nogales, B. S. Hsiao, R. H. Somani, S. Srinivas, A. H. Tsou, F. J. Balta-Calleja and T. A. Ezquerra, Polymer, 2001, 5247–5256 CrossRef CAS.
- J. Varga, J. Macromol. Sci., Part B: Phys., 2002, 41, 1121–1171 CrossRef PubMed.
- S. G. Markus Blomenhofer, D. Hanft, H.-W. Schmidt, M. Kristiansen, P. Smith, K. Stoll, D. Mader and K. Hoffmann, Macromolecules, 2005, 38, 3688–3695 CrossRef.
- J. Kang, H. Peng, B. Wang, Z. Chen, J. Li, J. Chen, Y. Cao, H. Li, F. Yang and M. Xiang, J. Appl. Polym. Sci., 2014, 131, 40115 CrossRef.
- D. Libster, A. Aserin and N. Garti, Polym. Adv. Technol., 2007, 18, 685–695 CrossRef CAS.
- H. Peng, B. Wang, J. Gai, J. Chen, F. Yang, Y. Cao, H. Li, J. Kang and M. Xiang, J. Appl. Polym. Sci., 2014, 131, 40027 Search PubMed.
- J. Kang, B. Wang, H. Peng, J. Li, J. Chen, J. Gai, Y. Cao, H. Li, F. Yang and M. Xiang, Polym. Adv. Technol., 2014, 25, 97–107 CrossRef CAS.
- J. Kang, Z. Chen, T. Zhou, F. Yang, J. Chen, Y. Cao and M. Xiang, J. Polym. Res., 2014, 21(384), 1–12 Search PubMed.
- Z. Chen, B. Wang, J. Kang, H. Peng, J. Chen, F. Yang, Y. Cao, H. Li and M. Xiang, Polym. Adv. Technol., 2014, 25, 353–363 CrossRef CAS.
- J. Kang, J. Gai, J. Li, S. Chen, H. Peng, B. Wang, Y. Cao, H. Li, J. Chen, F. Yang and M. Xiang, J. Polym. Res., 2013, 20(70), 1–11 CAS.
- A. Pawlak and E. Piorkowska, Colloid Polym. Sci., 2001, 279, 939–946 CAS.
- Q. Liu, X. Sun, H. Li and S. Yan, Polymer, 2013, 54, 4404–4421 CrossRef CAS PubMed.
- H. Li and S. Yan, Macromolecules, 2011, 44, 417–428 CrossRef CAS.
- H. Li, X. Sun, S. Yan and J. M. Schultz, Macromolecules, 2008, 41, 5062–5064 CrossRef CAS.
- S. Cheng, W. Hu, Y. Ma and S. Yan, Polymer, 2007, 48, 4264–4270 CrossRef CAS PubMed.
- H. Li, S. Jiang, J. Wang, D. Wang and S. Yan, Macromolecules, 2003, 36, 2802–2807 CrossRef CAS.
- D. Cavallo, G. Portale, L. Balzano, F. Azzurri, W. Bras, G. W. Peters and G. C. Alfonso, Macromolecules, 2010, 43, 10208–10212 CrossRef CAS.
- D. Cavallo, F. Azzurri, L. Balzano, S. r. S. Funari and G. C. Alfonso, Macromolecules, 2010, 43, 9394–9400 CrossRef CAS.
- G. C. Alfonso and F. Azzurri, Macromolecules, 2005, 38, 1723–1728 CrossRef.
- B. Zhang, J. Chen, F. Ji, X. Zhang, G. Zheng and C. Shen, Polymer, 2012, 53, 1791–1800 CrossRef CAS PubMed.
- B. Zhang, J. Chen, J. Cui, H. Zhang, F. Ji, G. Zheng, B. Heck, G. Reiter and C. Shen, Macromolecules, 2012, 45, 8933–8937 CrossRef CAS.
- J. C. Wittmann and B. Lotz, Prog. Polym. Sci., 1990, 15, 909–948 CrossRef CAS.
- Z. Zhang, Y. Tao, Z. Yang and K. Mai, Eur. Polym. J., 2008, 44, 1955–1961 CrossRef CAS PubMed.
- J. Varga and A. Menyhard, Macromolecules, 2007, 40, 2422–2431 CrossRef CAS.
- F. Luo, K. Wang, N. Ning, C. Geng, H. Deng, F. Chen, Q. Fu, Y. Qian and D. Zheng, Polym. Adv. Technol., 2011, 22, 2044–2054 CrossRef CAS.
- F. Luo, C. Geng, K. Wang, H. Deng, F. Chen, Q. Fu and B. Na, Macromolecules, 2009, 42, 9325–9331 CrossRef CAS.
- M. Dong, Z. Guo, Z. Su and J. Yu, J. Appl. Polym. Sci., 2011, 119, 1374–1382 CrossRef CAS.
- Y. Yamamoto, Y. Inoue, T. Onai, C. Doshu, H. Takahashi and H. Uehara, Macromolecules, 2007, 40, 2745–2750 CrossRef CAS.
- J. Kang, J. Chen, Y. Cao and H. Li, Polymer, 2010, 51, 249–256 CrossRef CAS PubMed.
- A. Khare, A. Mitra and S. Radhakrishnan, J. Mater. Sci., 1996, 31, 5691–5695 CrossRef CAS.
- J. Varga, J. Mater. Sci., 1992, 27, 2557–2579 CrossRef CAS.
- R. H. Olley, D. C. Bassett and D. J. Blundell, Polymer, 1986, 27, 344–348 CrossRef CAS.
- N. Pasquini and A. Addeo, Polypropylene Handbook, Hanser, 2005 Search PubMed.
- K. Wang, C. Zhou, C. Tang, Q. Zhang, R. Du, Q. Fu and L. Li, Polymer, 2009, 50, 696–706 CrossRef CAS PubMed.
- J. Scudla, M. Raab, K.-J. Eichhorn and A. Strachota, Polymer, 2003, 44, 4655–4664 CrossRef CAS.
- J. Kotek, I. Kelnar, J. Baldrian and M. Raab, Eur. Polym. J., 2004, 40, 679–684 CrossRef CAS PubMed.
- J. X. Li, W. L. Cheung and C. M. Chan, Polymer, 1999, 40, 2089–2102 CrossRef CAS.
- M. Dong, Z. Guo, J. Yu and Z. Su, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1725–1733 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |









