Bio-based semi-aromatic polyamide/functional clay nanocomposites: preparation and properties†
Meisam Shabanianbc,
Nianjun Kanga,
Jianwen Liub,
Udo Wagenknechtb,
Gert Heinrichbd and
De-Yi Wang*a
aMadrid Institute for Advanced Studies of Materials (IMDEA Materials), C/Eric Kandel, 2, 28906 Getafe, Madrid, Spain. E-mail: deyi.wang@imdea.org
bLeibniz-Institut für Polymerforschung Dresden e.V., Hohe Strasse 6, D-01069 Dresden, Germany
cFaculty of Chemistry and Petrochemical Engineering, Standard Research Institute (SRI), P.O. Box 31745-139, Karaj, Iran
dTechnische Universität Dresden, Institut für Werkstoffwissenschaft, D-01069 Dresden, Germany
First published on 2nd May 2014
Abstract
In this paper we first describe the design and synthesis of two novel cationic functional modifiers, i.e. a functionalized β-cyclodextrin (β-CD) derivative and tris(3-aminophenyl)phenyl phosphine oxide (TAP). Cloisite Na+ (clay-Na) and the modifiers were used for the preparation of the organoclay containing phosphine oxide (clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and of the organoclay containing β-cyclodextrin (clay-CD) via ion-exchange reaction. Biobased semi-aromatic polyamide (BPA)/functional clay (clay-P
O) and of the organoclay containing β-cyclodextrin (clay-CD) via ion-exchange reaction. Biobased semi-aromatic polyamide (BPA)/functional clay (clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and clay-CD) nanocomposites subsequently were prepared via solution blending. Effects of the two different types of organoclays on the flammable, thermal and mechanical properties of these biobased semi-aromatic polyamide nanocomposites were then studied. The properties of the nanocomposites were found to be strongly related to the nature of the modifiers. The clay-CD based nanocomposites (BPACD) showed more enhancements in thermal stability. The modifier containing the phosphine oxide moiety and triamine groups had stronger interactions with the polymer matrix, and exhibited superior mechanical properties, good flame retardancy and high thermal stability. Thus, we provide a new approach for comprehensive improvement of the properties of these bio-based semi-aromatic polyamide nanocomposite materials.
O and clay-CD) nanocomposites subsequently were prepared via solution blending. Effects of the two different types of organoclays on the flammable, thermal and mechanical properties of these biobased semi-aromatic polyamide nanocomposites were then studied. The properties of the nanocomposites were found to be strongly related to the nature of the modifiers. The clay-CD based nanocomposites (BPACD) showed more enhancements in thermal stability. The modifier containing the phosphine oxide moiety and triamine groups had stronger interactions with the polymer matrix, and exhibited superior mechanical properties, good flame retardancy and high thermal stability. Thus, we provide a new approach for comprehensive improvement of the properties of these bio-based semi-aromatic polyamide nanocomposite materials.
1. Introduction
In anticipation of the growing need for sustainable materials for the next century, utilization of natural renewable resources becomes an important consideration.1 Various materials from nontoxic plant/crop-based resources and biobased renewable products are strategic options that may be considered as alternatives to petroleum-based products. The most widely used renewable resource materials include polysaccharides, vegetable oils, wood and proteins. Various materials have been prepared from these naturally occurring renewable resources.2–5 Cyclodextrins belong to the family of cyclic homologous oligosaccharides that are obtained from the enzymatic degradation of starch. Cyclodextrins are composed of six or more α-D-glucopyranose units that are joined at the 1,4 positions by α-glucosidic linkages.6 The most commonly used natural cyclodextrin subtype is β-cyclodextrin, which consists of seven glucose units.Bio-composites provide an option for the use of new, high performance, “green” composite materials instead of conventional petroleum-based plastic materials. In the preparation of bio-composites and/or polymer nanocomposites, organically modified montmorillonites (O-MMT) are most widely used.7–10 Clays are generally organomodified or functionalized in order to form strong interactions between the polymer matrix and nanoclays. Organomodification of clay-Na+ is very important for the nanocomposites, since interactions between the polymer matrix and the nanoclays significantly influence the comprehensive properties of polymer nanocomposites. Fornes et al.11 investigated the properties of polyamide-6 (PA6)/montmorillonite by using different organic modifiers, and found that the surfactant structure affected the morphology and properties of nylon 6 nanocomposites. It is found that most conventional modifiers of clay are flammable, and that the flammability of these materials ultimately limits the flame retardant properties of polymer nanocomposites. Therefore, modifiers with low flammability and strong interactive properties become very important in the development of high performance clay based polymer nanocomposites. As an example of this, Wang et al. have developed a β-cyclodextrin-based layered double hydroxide (LDH) material with low flammability.12
Aromatic polyamides (a group of versatile high-performance polymers) display a wide range of applications and properties, and are useful for advanced technologies.13,14 However, their low solubility and high glass transition temperature often provide problems with their application. In order to address these problems, the use of semi-aromatic polyamides (synthesized via a combination of aliphatic and aromatic monomers) is regarded as a promising alternative. Semi-aromatic polyamides are generally exploited to fill the “performance gap” between high-performance polymers and nylons such as PA6.15 Such polyamides offer a wide range of properties including transparency, outstanding strength-to-weight ratios, high thermal resistance, and good barrier and solvent resistant properties, respectively.16–21 In our latest work, a novel biobased semi-aromatic polyamide (BPA) derived from nonanedioic acid and aromatic diamine containing a pyridine group has been synthesized and characterized.22 However, it has been found that while the use of conventional nanoclay (Cloisite 30B) can slightly improve the flame retardancy of BPA materials, it also severely decreases their mechanical properties.
In order to obtain high performance biobased semi-aromatic polyamide (BPA) nanocomposites, two new nanoclays were synthesized and characterized by using two functional modifiers containing modified phosphine oxide and modified β-cyclodextrin, respectively. BPA/organoclay nanocomposites then were prepared via solution blending. Effects of the two new organoclays on the morphology, thermal, flammability and mechanical properties of the BPA nanocomposites were investigated, and our findings are reported below.
2. Experimental
2.1 Materials
β-cyclodextrin, allyl glycidyl ether (AGE) and (3-chloro-2-hydroxypropyl)trimethylammonium chloride (CMA) were obtained from ABCR GmbH & Co. KG in Germany, and used without further purification. Triphenylphosphine oxide, palladium charcoal, N-methyl-2-pyrrolidone (NMP), N,N-dimethylacetamide (DMAc), hydrazine hydrate, pyridine, nitric acid, sulfuric acid, methanol, triphenyl phosphite (TPP), oleic acid, 4-nitroacetophenone, 4-hydroxybenzaldehyde, ammonium acetate, potassium permanganate (KMnO4) and glacial acetic acid obtained from Aldrich were used without further purification. Sodium montmorillonite (NaMMT; unmodified having CEC = 92.6 meq. per 100 g clay) from Southern Clay Products, Inc. was used.Commercially available calcium chloride (CaCl2, Aldrich) was dried under vacuum at 150 °C for 6 hours.
2.2 Synthesis of semi-aromatic biobased polyamide (BPA)22
2.3 Synthesis of β-cyclodextrin based modifier (CD-DB-N+) and tris(3-aminophenyl)phenyl phosphine oxide based modifier
In a 50 mL round-bottomed flask, 4.54 g β-CD (4 mmol) was added with stirring to an aqueous solution composed of 15 mL water and 3.0 g NaOH maintained at 70 °C. Subsequently, 1.83 g (16 mmol) AGE was slowly added, and the mixture was stirred for 6 hours. Following this, 5.45 g (40 mmol) CMA solution was slowly added with vigorous stirring. After the reaction was complete, the solution was cooled to room temperature and diluted with 30 mL water. The resulting solution was neutralized with 1 M hydrochloric acid and dialyzed against 3 × 700 mL water to remove salts (3-allyloxy-1,2-propanediol and hydroxyalkyl ammonium, respectively) formed as side products. The dialysate was then poured out into 100 mL of ethanol. The precipitate was filtered and dried under vacuum at 80 °C for 12 hours.Triamine containing phosphorus was synthesized in the laboratory according to the procedure reported elsewhere.24
2.4 Functional modification of nanoclays
Clay-CD was prepared by a cation exchange method. Clay-Na (5 g) was dispersed in 700 mL distilled water at room temperature. Another solution containing 2 g of CD-DB-N+ in 40 mL distilled water was prepared using magnetic stirring, and with the addition of 1.0 M HCl aqueous solution to adjust the pH value to 3–4. This solution was added to the clay suspension at a rate of approximately 15 mL min−1 with vigorous stirring. The mixture was stirred overnight at room temperature. Afterward, the organoclay was filtering using a Buchner funnel. The resulting organoclay (clay-CD) was washed with distilled water to remove any excess ammonium ions. The same procedure was used to prepare the organoclay based on the cationic form of TAP (clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2.5 Preparation of BPA/organoclay nanocomposites
The nanocomposites were synthesized by taking the BPA solution in a flask, followed by the addition of an appropriate amount of the organoclay at particular concentrations (see Scheme 1). For example, to prepare a nanocomposite containing 2 wt% of clay-CD, 0.98 g of the BPA was dissolved in DMAc in a 50 mL flask, followed by the addition of 0.02 g of clay-CD. The reaction mixture was agitated at high speed at 25 °C overnight to disperse clay platelets uniformly in the BPA matrix. The amount of clay-CD was 2 and 4 wt% in the nanocomposites (BPACD 2 and BPACD 4), respectively. By casting the suspension onto a glass plate followed by solvent evaporation in a vacuum oven, the final BPA nanocomposite films were prepared. The nanocomposites with the BPA and clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (BPAPO 2 and BPAPO 4) were prepared using the same procedure.
O (BPAPO 2 and BPAPO 4) were prepared using the same procedure.
2.6 Characterization
1H-NMR measurements were performed with a Bruker Avance III 500 spectrometer (Rheinstetten, Germany) operating at 500 MHz. DMSO-d6 was used as the solvent and the solvent signal was used for internal calibration (DMSO-d6: δ(1H) = 2.5 ppm).Morphological analysis was carried out using a LEO 912 transmission electron microscope. The conditions used during analysis were room temperature, 120 kV acceleration voltage, and bright field illumination.
Wide angle X-ray scattering (WAXS) was performed using a 2-circle diffractometer XRD 3003 θ/θ (GE Inspection Technologies/Seifert-FPM, Freiberg, Germany) with Cu-Kα radiation (λ = 0.154 nm) generated at 30 mA and 40 kV in the range of 2θ = 2–12° using 0.05° as the step length.
Thermal stability of the samples was investigated by thermogravimetric analysis (TGA; TA instruments Q 5000) in the range between room temperature and 800 °C at a heating rate of 10 °C min−1 in a nitrogen atmosphere. The glass transition temperatures of samples were measured by differential scanning calorimetry (DSC, TA instrument Q1000) in the range between 80 °C and 230 °C at a heating rate of 10 °C min−1 in a nitrogen atmosphere.
Microscale combustion calorimetry (MCC-1, FTT) is a convenient and relatively new technique developed in recent years for investigating the flammability of polymers.25,26 Using this technique, samples of approximately 5 mg mass were heated to 700 °C at a heating rate of 1 °C s−1 in a stream of nitrogen flowing at 80 cm3 min−1. The volatile, anaerobic thermal degradation products were then mixed with a 20 cm3 min−1 gas stream containing 20% oxygen and 80% nitrogen, respectively, prior to entering a 900 °C combustion furnace.
Tensile testing of the films was performed with FAVIGRAPH semiautomatic instrumentation (Textechno Company, Germany), equipped with a 100 N load cell. Specimens of 20 mm gauge length, 40–60 μm thickness, and 3 mm width were tested at a speed of 10 mm min−1. Relative humidity was kept at 50% and the temperature at 23 °C. Modulus strain between 0.1−0.2% was determined by stress–strain curve analysis.
3. Results and discussion
3.1 Synthesis and characterization of functional modifiers
The modified β-cyclodextrin was obtained by the reaction of hydroxyl groups in β-cyclodextrin with the electrophilic groups of AGE and CMA via a two-step reaction (Scheme 2). Structure conformation was determined by means of 1H NMR. As shown in Fig. 1, the singlet peak at 5.87 ppm was assigned to the proton on the carbon carbon double bonds (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) on the structure of AGE segments (corresponding to C1 in Fig. 1), while the peak at 4.21 ppm was assigned to protons on the CMA segment (corresponding to C3 in Fig. 1). All these NMR characteristic peaks matched the expected CD-DB-N+ structure. During the reaction, the protons of C2 on β-CD (Fig. 2) were not affected. For this reason, the ratio of the integrated peak for C2–H over that of C1–H at 5.87 ppm and C4–H at 3.25 ppm was used to estimate the number of carbon carbon double bonds and cationic segments grafted. By calculation, the ratio of C4–H/C2–H was 7.28, indicating that 5.66 cationic segments were attached per β-CD molecule. The ratio of C1–H/C2–H was 0.75, indicating that 5.25 carbon carbon double bonds were attached per β-CD molecule.
CH2) on the structure of AGE segments (corresponding to C1 in Fig. 1), while the peak at 4.21 ppm was assigned to protons on the CMA segment (corresponding to C3 in Fig. 1). All these NMR characteristic peaks matched the expected CD-DB-N+ structure. During the reaction, the protons of C2 on β-CD (Fig. 2) were not affected. For this reason, the ratio of the integrated peak for C2–H over that of C1–H at 5.87 ppm and C4–H at 3.25 ppm was used to estimate the number of carbon carbon double bonds and cationic segments grafted. By calculation, the ratio of C4–H/C2–H was 7.28, indicating that 5.66 cationic segments were attached per β-CD molecule. The ratio of C1–H/C2–H was 0.75, indicating that 5.25 carbon carbon double bonds were attached per β-CD molecule.
TAP was synthesized from triphenyl phosphine oxide, and first converted to tris(3-nitrophenyl)phenyl phosphine oxide by using concentrated nitric acid in the presence of sulfuric acid. Tris(3-nitrophenyl)phenyl phosphine oxide was then reduced to TAP with Pd/C and NH2NH2 (Scheme 1).
1H NMR (500 MHz, DMSO) δ, ppm of TAP: 7.10–7.14 (m, 3H), 6.84–6.87 (d, 3H), 6.71–6.73 (d, 3H), 6.62–6.65 (q, 4H), 5.33 (s, 6H).
3.2 Characterization of the nanocomposites
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. An increase of the interlayer distance led to a shift of the reflection and an increase in the basal spacing, providing evidence that intercalation had occurred. Similarly, it was noted that the d-spacing of clay-CD was 1.42 nm which was calculated from the reflection at 2θ = 6.2°. These findings indicate that the functional nanoclays, clay-P
O. An increase of the interlayer distance led to a shift of the reflection and an increase in the basal spacing, providing evidence that intercalation had occurred. Similarly, it was noted that the d-spacing of clay-CD was 1.42 nm which was calculated from the reflection at 2θ = 6.2°. These findings indicate that the functional nanoclays, clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and clay-CD, were prepared successfully.
O and clay-CD, were prepared successfully.
Fig. 3 also displays XRD patterns of BPA and corresponding nanocomposites. Composite films containing 2 and 4 wt%, respectively, of the two different organoclays showed no peak in the region 2θ = 2–12. No peaks in XRD patterns were observed for the BPA nanocomposites. This might mean that the organoclays dispersed homogeneously in the form of individual layers within the polymer matrix, or might be suggestive of the preferred orientation effect. Therefore, the absence of a diffraction peak could not be taken as proof for the formation of an exfoliated nanocomposite, and additional evidence based on transmission electron microscopy was required.
Thermal stability of the nanoclays was investigated under nitrogen conditions. Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTG) results of clay-Na+, clay-CD and clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are shown in Fig. 4. Compared to the thermal stability of clay-Na+, the modified clays (clay-CD, clay-P
O are shown in Fig. 4. Compared to the thermal stability of clay-Na+, the modified clays (clay-CD, clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) showed relatively low initial decomposition temperatures. Additionally, their char residues at 700 °C were very different. For clay-Na+, the weight loss between 450 and 700 °C corresponded to the condensation of silanol groups in the clay; however, for the modified clays, the weight loss between these temperatures was caused by the thermal degradation of the intercalated modifiers. As shown in Fig. 4, the char residue of clay-Na+ was 94.1 wt%, while the char residues of clay-CD and clay-P
O) showed relatively low initial decomposition temperatures. Additionally, their char residues at 700 °C were very different. For clay-Na+, the weight loss between 450 and 700 °C corresponded to the condensation of silanol groups in the clay; however, for the modified clays, the weight loss between these temperatures was caused by the thermal degradation of the intercalated modifiers. As shown in Fig. 4, the char residue of clay-Na+ was 94.1 wt%, while the char residues of clay-CD and clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were 77.4 wt% and 81.2 wt%, respectively. Therefore, if char formation of the organomodifiers at 700 °C is ignored, the amount of organomodifiers in clay-CD and clay-P
O were 77.4 wt% and 81.2 wt%, respectively. Therefore, if char formation of the organomodifiers at 700 °C is ignored, the amount of organomodifiers in clay-CD and clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are 16.7 wt% and 12.9 wt%, respectively.
O are 16.7 wt% and 12.9 wt%, respectively.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and clay-CD, the nanocomposites were more stable than BPA, showing significant increases at the 5 wt% weight loss temperature (T5) and the 10 wt% weight loss temperature (T10). T5 and T10 of all the nanocomposites were higher than those of the neat BPA. Considering the TGA and DTG curves of nanocomposites, we find that the organoclays had a significant effect on the thermal degradation process of BPA. The thermal stability of the nanocomposites increased due to the higher barrier/shielding effect resulting from the clay content in the matrix polymer. Both of the organoclays displayed excellent barrier effects to prevent thermal degradation of polymer chains at relatively low temperatures. The clay layers present in the BPA matrix restricted the diffusion of heat to its bulk, and thus delayed the degradation process. Upon inspection of the char residues of the samples at 800 °C, we note that overall, the nanocomposites slightly improved the char residue at high temperature.
O and clay-CD, the nanocomposites were more stable than BPA, showing significant increases at the 5 wt% weight loss temperature (T5) and the 10 wt% weight loss temperature (T10). T5 and T10 of all the nanocomposites were higher than those of the neat BPA. Considering the TGA and DTG curves of nanocomposites, we find that the organoclays had a significant effect on the thermal degradation process of BPA. The thermal stability of the nanocomposites increased due to the higher barrier/shielding effect resulting from the clay content in the matrix polymer. Both of the organoclays displayed excellent barrier effects to prevent thermal degradation of polymer chains at relatively low temperatures. The clay layers present in the BPA matrix restricted the diffusion of heat to its bulk, and thus delayed the degradation process. Upon inspection of the char residues of the samples at 800 °C, we note that overall, the nanocomposites slightly improved the char residue at high temperature.
| Samples | Tinitiala (°C) | T10b (°C) | Tmax1c (°C) | Tmax2 (°C) | Chard yield | Tge |
|---|---|---|---|---|---|---|
| a Temperature at which 5% weight loss was recorded by TGA at a heating rate of 10 °C min−1.b Temperature at which 10% weight loss was recorded by TGA at a heating rate of 10 °C min−1.c Maximum decomposition temperatures.d Weight percentage of material left after TGA analysis at a maximum temperature of 800 °C.e Glass transition temperature recorded at a heating rate of 10 °C min−1 in a nitrogen atmosphere. | ||||||
| BPA | 153 | 195 | 168 | 409 | 58.3 | 130 |
| BPACD 2 | 188 | 236 | 205 | 444 | 59.8 | 165 |
| BPACD 4 | 195 | 246 | 205 | 447 | 60.5 | 138 |
| BPAPO 2 | 192 | 233 | 223 | 439 | 58.1 | 133 |
| BPAPO 4 | 194 | 243 | 205 | 439 | 60.2 | 148 |
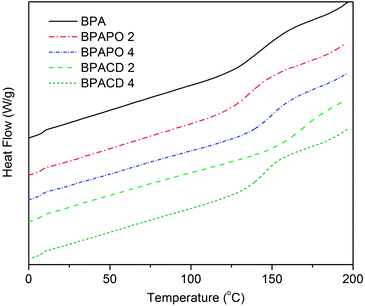
The glass transition temperature (Tg) of the neat BPA and the nanocomposites were investigated using differential scanning calorimetry (DSC), and the results are given in Table 1. The first heating run of DSC was ignored due to the influence of thermal annealing history, and thermal properties were evaluated according to the DSC curves of the second heating (Fig. 7). It was found that addition of the nanoclays caused an increase in the Tg. This suggests that movement of the BPA chains is restricted by the layers of organoclay, thereby increasing the Tg values of the nanocomposites. Among the two types of organoclay with different loading, BPACD 2 gave the greatest increase in Tg value (Tg = 165 °C) compared to neat BPA. The order of the Tg of the nanocomposites was BPACD 2 > BPAPO 4 > BPACD 4 > BPAPO 2 > BPA.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, both BPAPO 2 and BPAPO 4 exhibited even lower pHRR and THR values, indicating that the improvement of flame retardancy in this system was not completely due to the char yield (see Table 2). The decreased pHRR and THR with a small amount of clay-P
O, both BPAPO 2 and BPAPO 4 exhibited even lower pHRR and THR values, indicating that the improvement of flame retardancy in this system was not completely due to the char yield (see Table 2). The decreased pHRR and THR with a small amount of clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was attributed to compounds with low volatility released during the degradation procedure. The best pHRR and THR values were obtained by adding 4 wt% clay-P
O was attributed to compounds with low volatility released during the degradation procedure. The best pHRR and THR values were obtained by adding 4 wt% clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (pHRR = 38.9 W g−1 and THR = 8.6 kJ g−1). In addition to the impact of char residues (condensed phase mechanism) on the flame retardancy of BPACD and BPAPO, another flame retardant mechanism might be possible. The phosphine oxide moieties in the clay-P
O (pHRR = 38.9 W g−1 and THR = 8.6 kJ g−1). In addition to the impact of char residues (condensed phase mechanism) on the flame retardancy of BPACD and BPAPO, another flame retardant mechanism might be possible. The phosphine oxide moieties in the clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O might release some phosphorus-containing radicals which can capture the H˙ and HO˙ in the gas phase, so that the flammability of BPA nanocomposites remains low. This would explain why the flammability of BPAPO was always lower than that of BPACD.
O might release some phosphorus-containing radicals which can capture the H˙ and HO˙ in the gas phase, so that the flammability of BPA nanocomposites remains low. This would explain why the flammability of BPAPO was always lower than that of BPACD.
| Samples | BPA | BPACD 2 | BPACD 4 | BPAPO 2 | BPAPO 4 |
|---|---|---|---|---|---|
| pHRR (W g−1) | 47.6 | 43.3 | 42.1 | 40.2 | 38.9 |
| THR (kJ g−1) | 14.7 | 12.4 | 11.4 | 9.4 | 8.6 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the BPA matrix, providing more stiffness to the materials (stiffness being a function of the aspect ratio). Such improvement of the mechanical properties of BPAPO may be attributed to the extensive interaction (via formation of hydrogen bonding) between the triamine TAP modifier in BPAPO and the BPA chains. The results also indicate significant increase in tensile strength of BPAPO and BPACD, compared with that of the neat BPA. The incorporation of these functional organoclays into the polymer chains can reinforce the polymer, giving the observed increase in this important property. When using conventional modified nanoclay (Cloisite 30B), mechanical properties of BPA nanocomposites (e.g. tensile strength at break) are severely decreased.22 Intimate blending of the two phases, presumably with chemical bonding between them, provides a combination of some of the best properties of the two components.27 The maximum stress at break (ultimate strength) was found to increase initially with increase in clay-P
O in the BPA matrix, providing more stiffness to the materials (stiffness being a function of the aspect ratio). Such improvement of the mechanical properties of BPAPO may be attributed to the extensive interaction (via formation of hydrogen bonding) between the triamine TAP modifier in BPAPO and the BPA chains. The results also indicate significant increase in tensile strength of BPAPO and BPACD, compared with that of the neat BPA. The incorporation of these functional organoclays into the polymer chains can reinforce the polymer, giving the observed increase in this important property. When using conventional modified nanoclay (Cloisite 30B), mechanical properties of BPA nanocomposites (e.g. tensile strength at break) are severely decreased.22 Intimate blending of the two phases, presumably with chemical bonding between them, provides a combination of some of the best properties of the two components.27 The maximum stress at break (ultimate strength) was found to increase initially with increase in clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content, and at 4 wt% organoclay, showed a maximum value of 69.5 MPa. Relative to the corresponding 59.4 MPa value for neat BPA, this represents significant enhancement.
O content, and at 4 wt% organoclay, showed a maximum value of 69.5 MPa. Relative to the corresponding 59.4 MPa value for neat BPA, this represents significant enhancement.
| Samples | Modulus [GPa] (ε 0.1–0.2%) | Maximum stress σm [MPa] | Strain at σm εm [%] | Tensile strength at break σb [MPa] | Strain at σb εb [%] | Work to fracture W [MJ m−3] |
|---|---|---|---|---|---|---|
| BPA | 2.2 ± 0.1 | 59.4 ± 6.5 | 3.6 ± 0.5 | 54.6 ± 5.5 | 4.0 ± 0.8 | 1.5 ± 0.4 |
| BPACD 2 | 2.1 ± 0.1 | 58.2 ± 6.2 | 3.7 ± 0.5 | 58.0 ± 6.0 | 3.7 ± 0.6 | 1.3 ± 0.4 |
| BPACD 4 | 2.6 ± 0.1 | 61.4 ± 4.1 | 3.2 ± 0.4 | 61.3 ± 4.1 | 3.2 ± 0.4 | 1.2 ± 0.2 |
| BPAPO 2 | 2.1 ± 0.1 | 62.8 ± 3.5 | 4.1 ± 0.3 | 62.6 ± 3.5 | 4.1 ± 0.3 | 1.5 ± 0.2 |
| BPAPO 4 | 3.0 ± 0.2 | 69.5 ± 6.6 | 3.3 ± 0.4 | 69.5 ± 6.5 | 3.3 ± 0.5 | 1.4 ± 0.3 |
4. Conclusions
Since design and selection of functional nanoclays are very important for preparing polymer nanocomposites, two novel organoclays containing modified phosphine oxide (TAP) and functional β-CD were synthesized via ion-exchange reaction. Two types of BPA nanocomposites, BPACD and BPAPO, were produced by solution blending. TEM results indicated the formation of a non-homogeneous distribution in some orientations of the nanocomposites. Thermal stability of BPA nanocomposites was improved compared with neat BPA, and in particular, clay-CD based nanocomposites showed more improvements. The improvement in thermal stability of BPA nanocomposites was attributed primarily to the physical barrier effect of the organoclays. Flammability of the BPA nanocomposites with different nanoclay concentrations was investigated by microscale combustion calorimetry (MCC). It was found that clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O had the best effect on improving the flame retardancy of BPA, showing the lowest values of peak heat release rate (pHRR) and total heat release (THR) of the BPA nanocomposites. With the increase of clay-P
O had the best effect on improving the flame retardancy of BPA, showing the lowest values of peak heat release rate (pHRR) and total heat release (THR) of the BPA nanocomposites. With the increase of clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O loading, the BPA nanocomposites showed improved flame retardancy. In the case of clay-CD based nanocomposites, mechanical properties and flame retardancy of BPACD also increased with the increase of nanoclay loading. In comparison, clay-P
O loading, the BPA nanocomposites showed improved flame retardancy. In the case of clay-CD based nanocomposites, mechanical properties and flame retardancy of BPACD also increased with the increase of nanoclay loading. In comparison, clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O based nanocomposites showed even greater improvements in flame retardancy and mechanical properties. Besides the char residue impact on flame retardancy of both BPACD and BPAPO, another possible flame retardant mechanism for BPAPO involves phosphine oxide moieties in the clay-P
O based nanocomposites showed even greater improvements in flame retardancy and mechanical properties. Besides the char residue impact on flame retardancy of both BPACD and BPAPO, another possible flame retardant mechanism for BPAPO involves phosphine oxide moieties in the clay-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O releasing some phosphorus-containing radicals which can capture the H˙ and HO˙ in the gas phase, so that the flammability of BPA nanocomposites remains low. Here, we have systematically investigated and described the effects of functional organoclays on flammability, thermal and mechanical properties of BPA nanocomposites. Our findings strongly suggest that these sustainable biobased semi-aromatic polyamide/functional clay nanocomposites have great potential for being used to develop high performance materials in different areas.
O releasing some phosphorus-containing radicals which can capture the H˙ and HO˙ in the gas phase, so that the flammability of BPA nanocomposites remains low. Here, we have systematically investigated and described the effects of functional organoclays on flammability, thermal and mechanical properties of BPA nanocomposites. Our findings strongly suggest that these sustainable biobased semi-aromatic polyamide/functional clay nanocomposites have great potential for being used to develop high performance materials in different areas.
Acknowledgements
This research is partly funded by the European Commission under the 7th Framework Programme (Marie Curie Career Integration Grant) and Ramón y Cajal grant.Notes and references
- Y. Parulekar and A. K. Mohanty, Green Chem., 2006, 8, 206–213 RSC.
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- Y. Xia and R. C. Larock, Green Chem., 2010, 12, 1893–1909 RSC.
- J. Zuo, S. Li, L. Bouzidi and S. S. Narine, Polymer, 2011, 52, 4503–4516 CrossRef CAS PubMed.
- M. Haq, R. Burgueño, A. K. Mohanty and M. Misra, Composites, Part A, 2009, 40, 540–547 CrossRef PubMed.
- F. van de Manakker, T. Vermonden, C. F. van Nostrum and W. E. Hennink, Biomacromolecules, 2009, 10, 3157–3175 CrossRef CAS PubMed.
- C. A. Diaz, Y. Xia, M. Rubino, R. Auras, K. Jayaraman and J. Hotchkiss, Nanoscale, 2013, 5, 164–168 RSC.
- T. Jiang, Y.-h. Wang, J.-t. Yeh and Z.-q. Fan, Eur. Polym. J., 2005, 41, 459–466 CrossRef CAS PubMed.
- D. Y. Wang, U. Gohs, N. J. Kang, A. Leuteritz, R. Boldt, U. Wagenknecht and G. Heinrich, Langmuir, 2012, 28(34), 12601–12608 CrossRef CAS PubMed.
- D. Y. Wang, Y. P. Song, J. S. Wang, X. G. Ge, Y. Z. Wang, A. A. Stec and T. R. Hull, Nanoscale Res. Lett., 2009, 4, 303–306 CrossRef CAS PubMed.
- T. D. Fornes, P. J. Yoon, D. L. Hunter, H. Keskkula and D. R. Paul, Polymer, 2002, 43, 5915–5933 CrossRef CAS.
- N. J. Kang and D. Y. Wang, J. Mater. Chem. A, 2013, 1(37), 11376–11383 CAS.
- M. Trigo-Lopez, P. Estevez, N. San-Jose, A. Gomez-Valdemoro, F. C. Garcia, F. Serna, J. L. d. l. Pena and J. M. Garcia, Recent Pat. Mater. Sci., 2009, 2, 190–208 CrossRef CAS.
- M. Shabanian and N. Basaki, Composites, Part B, 2013, 52, 224–232 CrossRef CAS PubMed.
- S. Zulfiqar, A. Kausar, M. Rizwan and M. I. Sarwar, Appl. Surf. Sci., 2008, 255, 2080–2086 CrossRef CAS PubMed.
- A. S. Patil, M. Medhi, N. V. Sadavarte, P. P. Wadgaonkar and N. N. Maldar, Mater. Sci. Eng., B, 2010, 168, 111–116 CrossRef CAS PubMed.
- A. L. Chen, K. L. Wei, R. J. Jeng, J. J. Lin and S. A. Dai, Macromolecules, 2010, 44, 46–59 CrossRef.
- Y. Ito, T. Higashihara and M. Ueda, Macromolecules, 2012, 45, 4175–4183 CrossRef CAS.
- S. Shabbir, S. Zulfiqar, Z. Ahmad and M. I. Sarwar, Tetrahedron, 2010, 66, 7204–7212 CrossRef CAS PubMed.
- M. Shabanian, N. J. Kang, D. Y. Wang, U. Wagenknecht and G. Heinrich, Polym. Degrad. Stab., 2013, 98, 1036–1042 CrossRef CAS PubMed.
- M. Shabanian, N. Kang, D. Y. Wang, U. Wagenknecht and G. Heinrich, RSC Adv., 2013, 3, 20738–20745 RSC.
- M. Shabanian, H. Moghanian, J. W. Liu, U. Wagenknecht, G. Heinrich and D. Y. Wang, Novel Aliphatic–Aromatic Polyamide Nanocomposite Derived from Biobased Diacid: Synthesis and Characterization, Chem. Mater., 2013 Search PubMed , Submitted.
- J. W. Hill and W. L. McEwen, Org. Synth., 1933, 13, 4 CrossRef CAS.
- I. K. Varma, G. M. Fohlen and J. A. Parker, J. Macromol. Sci., Part A: Pure Appl.Chem., 1983, 19, 209–224 CrossRef.
- Y. Shi, T. Kashiwagi, R. N. Walters, J. W. Gilman, R. E. Lyon and D. Y. Sogah, Polymer, 2009, 50, 3478–3487 CrossRef CAS PubMed.
- D. Y. Wang, A. Das, F. R. Costa, A. Leuteritz, Y. Z. Wang, U. Wagenknecht and G. Heinrich, Langmuir, 2010, 26, 14162–14169 CrossRef CAS PubMed.
- S. Zulfiqar and M. Sarwar, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 353–361 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02949f |
| This journal is © The Royal Society of Chemistry 2014 |