Selective sensing of Al3+ by naphthyridine coupled rhodamine chemosensors†
Abstract
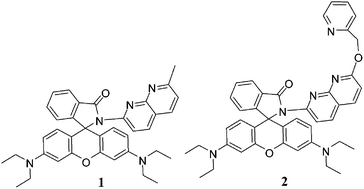
Napthyridine-based rhodamine chemosensors 1 and 2 are designed and synthesized. Both the chemosensors selectively recognize Al3+ ion over a series of other cations in CH3CN–H2O (4 : 1, v/v, 10 μM Tris–HCl buffer, pH = 7.0) by exhibiting change in color (colorless to pink) and emission at 588 nm in the turned on mode. Complexation is reversible and the ensembles of 1·Al3+ and 2·Al3+ selectively sense F− ion by discharging the pink color of the solutions and bringing a reverse change in both absorption and emission spectra.

 Please wait while we load your content...
Please wait while we load your content...