Deproto-metallation using mixed lithium–zinc and lithium–copper bases and computed CH acidity of 2-substituted quinolines†
Nada Marquisea,
Guillaume Bretela,
Frédéric Lassagnea,
Floris Chevallier*a,
Thierry Roisnelb,
Vincent Dorcetb,
Yury S. Halauko*c,
Oleg A. Ivashkevichc,
Vadim E. Matulisd,
Philippe C. Grose and
Florence Mongin*a
aChimie et Photonique Moléculaires, Institut des Sciences Chimiques de Rennes, UMR 6226 Université de Rennes 1, CNRS, Bâtiment 10A, Case 1003, Campus Scientifique de Beaulieu, 35042 Rennes, France. E-mail: floris.chevallier@univ-rennes1.fr; florence.mongin@univ-rennes1.fr; Fax: +33-2-2323-6955
bCentre de DIFfractométrie X, Institut des Sciences Chimiques de Rennes, UMR 6226 Université de Rennes 1, CNRS, Bâtiment 10B, Campus de Beaulieu, 35042 Rennes, France
cUNESCO Chair of Belarusian State University, 14 Leningradskaya Str., Minsk, 220030, Belarus. E-mail: hys@tut.by
dResearch Institute for Physico-Chemical Problems of Belarusian State University, 14 Leningradskaya Str., Minsk, 220030, Belarus. Fax: +375-17-2264696
eHECRIN, SRSMC, Université de Lorraine-CNRS, Boulevard des Aiguillettes, 54506, Vandoeuvre-Lès-Nancy, France
First published on 16th April 2014
Abstract
2-Substituted quinolines were synthesized, and their deproto-metallation using the bases prepared by mixing LiTMP with either ZnCl2·TMEDA (1/3 equiv.) or CuCl (1/2 equiv.) was studied. With phenyl and 2-naphthyl substituents, the reaction occurred at the 8 position of the quinoline ring, affording the corresponding iodo derivatives or 2-chlorophenyl ketones using the lithium–zinc or the lithium–copper combination, respectively. With a 4-anisyl substituent, a dideprotonation at the 8 and 3′ position was noted using the lithium–zinc base. With 3-pyridyl, 2-furyl and 2-thienyl substituents, the reaction took place on the substituent, at a position adjacent to its heteroatom. 2-Chlorophenyl-2-phenyl-8-quinolyl ketone could be cyclized under palladium catalysis. The experimental results were analyzed with the help of the CH acidities of the substrates, determined in THF solution using the DFT B3LYP method.
Introduction
Quinolines are key heterocycles present in numerous natural products and biologically active compounds.1 In addition, derivatives are widely used in different fields, e.g. as biocides, dyes, rubber chemicals and corrosion inhibitors.2 Deprotonative lithiation3 has been developed in the quinoline series,4 in general with recourse to substituents capable of directing the reaction to specific sites through acidifying and/or coordinating effects. Due to the low LUMO level of such substrates, very low temperatures are often required in the protocoles employed.Efficient tools for the deproto-metallation of sensitive aromatic compounds have recently emerged. In particular, synergic combinations of lithium reagents and softer metal compounds such as bimetal ate compounds, salt-activated metal amides, and pairs of metal amides have appeared as promising alternatives to lithium bases,5 and notably in the quinoline series.6
In the search of new bimetal ate bases, our group developed (TMP)2CuLi(±LiCl) (TMP = 2,2,6,6-tetramethylpiperidido),7 a lithiocuprate prepared in situ from CuCl and LiTMP. Besides its possible use at room temperature, one main advantage of using the base is the possible trapping of the formed arylmetal species by aroyl chlorides to directly afford ketones.
Our group also developed the use of the TMP-based pair of metal amides, in situ prepared by mixing ZnCl2·TMEDA (TMEDA = N,N,N',N'-tetramethylethylenediamine) with LiTMP (3 equiv.),8 and supposed to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LiTMP·2LiCl(±TMEDA)–Zn(TMP)2 mixture.9 By complementing each other in deproto-metallation reactions, this mixture of lithium and zinc amides allows sensitive substrates to be mono- or di- deproto-metallated, as evidenced by subsequent iodolysis.7c,8,10 In addition, compared with both heteroleptic amido-organo lithium zincates10d and salt-activated hindered zinc amides,5d the above combination allows the conversion of less activated substrates.
1 LiTMP·2LiCl(±TMEDA)–Zn(TMP)2 mixture.9 By complementing each other in deproto-metallation reactions, this mixture of lithium and zinc amides allows sensitive substrates to be mono- or di- deproto-metallated, as evidenced by subsequent iodolysis.7c,8,10 In addition, compared with both heteroleptic amido-organo lithium zincates10d and salt-activated hindered zinc amides,5d the above combination allows the conversion of less activated substrates.
Synthesis of 2-substituted quinolines, e.g. 2-arylquinolines, was recently simplified with the one-pot Friedländer access from 2-nitroarylcarbaldehydes.11 We thus decided to study the deprotonative metallation of the substrates 1 (Scheme 1) using both the lithium–zinc and the lithium–copper base in order to prepare iodo derivatives and ketones, respectively, after direct trapping. We earlier showed that the regioselectivity of the reactions for related substrates is partly determined by the acidity of the different hydrogens in their molecules.7c,10j Similarly, here we tried to rationalize the outcome of reactions by using the CH acidities in THF of the quinoline-based substrates calculated by means of the isodesmic reaction approach within the density functional theory (DFT) framework.
Results and discussions
Synthetic aspects
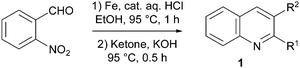
To reach the target quinolines 1, the conditions reported by Li, Mulvihill and co-workers were employed.11 Thus, 2-nitrobenzaldehyde was reduced using iron in the presence of catalytic aqueous HCl, and the obtained 2-aminobenzaldehyde was condensed in situ with ketones to form either 2-substituted or 2,3-disubstituted quinolines in high yields (Table 1). The structure of 1e was confirmed by X-ray diffraction analysis (Fig. 1, left).‡| Entry | Ketone | Product (R1, R2) | Yielda (%) |
|---|---|---|---|
| a After purification. | |||
| 1 | PhCOMe | 1a (Ph, H) | 99 |
| 2 | 2-NaphthylCOMe | 1b (2-naphthyl, H) | 90 |
| 3 | 4-MeOC6H4COMe | 1c (4-MeOC6H4, H) | 95 |
| 4 |  |
1d (C6H4-2-CH2) | 87 |
| 5 | 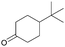 |
1e ((CH2)2C(t-Bu)CH2) | 91 |
| 6 | 3-PyridylCOMe | 1f (3-pyridyl, H) | 89 |
| 7 | 2-FurylCOMe | 1g (2-furyl, H) | 100 |
| 8 | 2-ThienylCOMe | 1h (2-thienyl, H) | 80 |
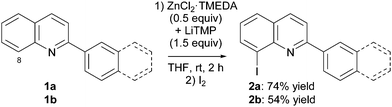
The lithium–zinc TMP-based mixture of amides, prepared in situ from ZnCl2·TMEDA (0.5 equiv.) and LiTMP (1.5 equiv.), was first attempted for the deproto-metallation of the quinolines 1. Previous studies aimed at optimizing the reaction on different substrates showed that THF is the most suitable solvent, and 2 h a sufficient reaction time for a reaction performed at room temperature.7c,10a,b In addition, iodolysis was similarly identified as an efficient quenching mode for the arylmetal compounds thus generated.10d
Upon treatment under these conditions, 2-phenyl and 2-naphthylquinoline 1a,b were converted to the corresponding 8-iodo derivatives 2a,b in 74 and 54% yield, respectively (Scheme 2). The product 2a was identified unequivocally by X-ray diffraction (Fig. 1, right).‡ Functionalization at the 8 position had previously been observed by deproto-lithiation4 or -magnesiation6f,12 of quinolines, but in general either in the presence of a directing group at the 7 position or in the absence of free/accessible position on the quinoline ring. For bare quinoline, deproto-metallation using the Kondo's TMP-zincate LiZn(TMP)(t-Bu)2 followed by iodolysis was reported, and showed the formation of both the 2- and 8-iodo regioisomers in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio.13 A substituted 2 position together with the absence of directing group on the pyridine ring leads to a reaction at the 8 position in the case of 1a,b.
7 ratio.13 A substituted 2 position together with the absence of directing group on the pyridine ring leads to a reaction at the 8 position in the case of 1a,b.
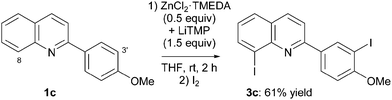
Anisole being easily ortho-deprotonated under similar reaction conditions,10d it was interesting to involve in the deprotonation–iodination sequence 2-(4-methoxyphenyl)quinoline (1c). Under the same reaction conditions, the diiodide 3c was obtained in 61% yield (Scheme 3). Such a double functionalization generally does not take place using monometal lithium bases, but is a reaction commonly observed using the present lithium–zinc base.10e,g,j
In the case of 11H-indeno[1,2-b]quinoline (1d), no more ring deprotonation was noted due to the presence of more acidic benzylic hydrogens. Under the same reactions conditions, the mono and diiodide 2d and 3d were isolated in 4 and 51% yield, respectively (Scheme 4). From 2-tert-butyl-1,2,3,4-tetrahydroacridine (1e), a complex mixture of iodides was formed, a result probably due to a larger number of deprotonation sites in this case.
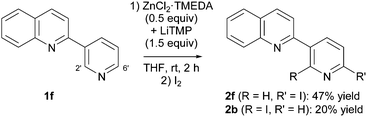
By switching from 2-phenylquinoline (1a) to 2-(3-pyridyl)quinoline (1f), the regioselectivity of the reaction was completely modified. The deproto-metallation no more took place on the quinoline ring but on its pyridine substituent. In addition, the two sites next to the pyridine ring were unregioselectively attacked, with a preference for the less hindered 6 position (product 2f, 47% yield) over the 2 position (product 2f′, 20% yield) (Scheme 5).
When submitted to the same reaction conditions, 2-(2-furyl)quinoline (1g) was attacked at the furan ring, next to oxygen, to afford the iodo derivative 2g in 71% yield (Scheme 6).
2-(2-Thienyl)quinoline (1h) had previously been deproto-lithiated using butyllithium in ethereal solvents, either to give a mixture resulting from a reaction at the 3′ or 5′ position at 0 °C (trapping with chlorotrimethylsilane),3a or to furnish the product functionalized at the 5′ position at −78 °C (interception by DMF).14
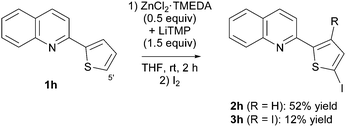
By using the lithium–zinc base as before, the bis(heterocycle) 1h was functionalized at the thienyl ring. The corresponding monoiodide 2h and diiodide 3h were isolated in 52 and 12% yield, respectively (Scheme 7 and Fig. 2).
In order to reach other kinds of functionalization by direct trapping after deproto-metallation, we turned to another base. It was decided to attempt the use of the lithiocuprate prepared in situ from CuCl and LiTMP (2 equiv.) on some of the quinoline substrates in order to prepare ketone derivatives by interception with an aroyl chloride.7
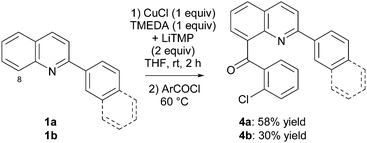
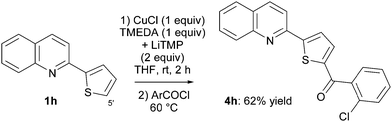
Previous studies established THF containing TMEDA (1 equiv.) as the most suitable solvent, and 2 h as a sufficient reaction time for a reaction performed at room temperature.7 First, the TMP-based lithiocuprate, prepared in situ from CuCl (1 equiv.) and LiTMP (2 equiv.), was evaluated for the deproto-metallation of 2-phenyl and 2-naphthylquinoline 1a,b. Both substrates were functionalized at their 8 position, affording after trapping with 2-chlorobenzoyl chloride the ketones 4a,b in 58 and 30% yield, respectively (for the latter, recovery of starting material was also noted) (Scheme 8 and Fig. 3). Similarly, 2-(2-thienyl)quinoline (1h) was converted to the ketone 4h, isolated in 62% yield (Scheme 9).
It is possible to involve the generated heterocyclic iodides in different transition metal-catalyzed coupling reactions,15 and the chloro ketones in intramolecular direct arylation through palladium-catalysed C–H bond activation.16 To attempt the conversion of 8-(2-chlorobenzoyl)-2-phenylquinoline (4a) to the fluorenone 5a, we proceeded as reported for the cyclization of 2-chloro diaryl aniline to carbazole.17 Using catalytic amounts of Pd(OAc)2, Cy3P (Cy = cyclohexyl) as an electron-rich and bulky trialkyl phosphine, K2CO3 as a base and DMF as solvent at 130 °C afforded the tetracycle 5a in a moderate 45% yield due to the competitive formation of 8-benzoyl-2-phenylquinoline in 30% yield (Scheme 10 and Fig. 4).
Computational aspects
Unfortunately, both experimental and computational data on CH acidity for quinoline and its derivatives are scanty. The main reasons are the necessity of using very strong bases at low temperatures, the possible side reactions of the generated carbanions and also the acidity closeness between different C–H bonds in such condensed aromatics. A brief review of papers, devoted to experimental and theoretical investigation of CH acidity in azines, is presented in our previous publication.7c For such aromatic substrates, one should especially mention the pKa values experimentally found for 4-methylquinoline and several alkylpyridines in THF,18 and for substituted arenes and fluorenes in CH3CN.19 Some experimental gas-phase acidities of condensed heteroaromatics including bare quinoline were also reported.20 To the long-standing semi-empirical studies of the deprotonation energies of nitrogen heterocycles,21 DFT calculations of pKa values of benzo-azines22 and -quinuclidines23 were recently added.In the present paper, the DFT calculations of CH acidity of the different quinolines, both in gas phase (see ESI†) and in THF solution (Scheme 11), are presented. The gas phase acidities ΔacidG and pKa values in THF solution of all the substrates were calculated using the theoretical protocol thoroughly described previously.24 This approach already proved to be worthy for substituted azines7c and biaryl compounds.10j
All the calculations were performed by using the DFT B3LYP method. The geometries were fully optimized using the 6-31G(d) basis set. No symmetry constraints were implied. In order to perform stationary points assessment and to calculate zero-point vibrational energies (ZPVE) and thermal corrections, the Hessian matrix eigenvalues were calculated at the same level of theory. The single point energies were found using the 6-311+G(d,p) basis set and tight convergence criteria. The gas phase Gibbs energies (G0298) were calculated for each isolated species using the following equation:
| G0298 = E + ZPVE + H0→298 − TS0298. |
The gas phase acidities ΔacidG were determined as the Gibbs energies of deprotonation of the substrates R–H (R–H(g) → R−(g) + H+(g)) by the following formula:
| ΔacidG = G0298(R−) + G0298(H+) − G0298(RH). |
The solvent effects were treated by using the polarized continuum model (PCM) with the default parameters for THF.25 The PCM energies EPCM were also calculated at the B3LYP/6-311+G(d,p) level using geometries optimized for isolated structures. The Gibbs energies in solution Gs were calculated for each species by the formula:
| Gs = G0298 + EPCM − E. |
To cancel the majority of calculation errors, the pKa values were calculated by means of the following isodesmic reaction:
| R–H(s) + Het−(s) → R−(s) + Het–H(s), |
It could be shown easily that the Gibbs energies of the isodesmic reactions (ΔrGs) and the corresponding pKa values are linked together by the following equation:
The CH acidity of the methoxy group for the substrate 1c was not considered here since it was expected to be significantly lower and there was no sign of its deprotonation in the experiment. The calculations on 1e were also skipped due to the obvious prevalence of benzylic acidity in deprotonation.
It is obvious that, with the exception of 1a and 1d, all the investigated arylquinolines exist in form of several rotamers. So, the data on Scheme 11 (and ESI†) refer to the most stable ones. Among these molecules with several rotamers, the naphthyl derivative 1b is likely to exist in the stretched form, 1f and 1g with remote heteroatoms, while for the sulfur-containing compound 1h the syn-form should prevail. These findings are in agreement with those for arylated azoles.10k
There are several potential deprotonation sites in the investigated substrates. When comparing the CH acidity in gas-phase (see ESI†) and in THF solution (Scheme 11), the correlation can be easily seen. The analysis of the obtained results shows that CH acidity increases logically with the introduction of electron-withdrawing groups, and decreases for electron-donating ones.
The calculated values of gas-phase acidity of the investigated quinolines mostly lie within the range of 375 to 390 kcal mol−1 (see ESI†). Such ΔacidG values are typical of very weak acids and correspond to those found previously in experiment for bare quinoline20 (exp. 376.9 kcal mol−1 vs. calc. 377.3 kcal mol−1, position 4) as well as for computed ones.22 When analyzing the pKa values distribution for a common quinolinic part of the molecules, one can see some general trends. The most acidic hydrogen is at the position 4, while the least acidic is at the 8.
Discussion
The calculations of the CH acidities in THF of 1a–d and 1f–h (Scheme 11) allowed us to comment on the regioselectivities observed in the course of the reactions involving these substrates.The calculations performed on the quinoline derivatives 1a,b show that the hydrogens at the two free positions of the pyrido ring are more acidic than those of the benzo and aryl rings. Nevertheless, reactions did not lead to any 3- and 4-substituted quinolines but to the 8-iodo and 8-(2-chlorobenzoyl) derivatives 2a,b and 4a,b, respectively using the lithium–zinc (Scheme 2) and lithium–copper (Scheme 8) bases. Such reactions at the 8 position could be due to the presence of a nitrogen able to coordinate a metal, either favouring the approach of the base and/or stabilizing the arylmetal compound formed. Recently, C–H borylation of quinoline derivatives was similarly observed at the C8 position.26
Using the lithium–zinc base under the same reaction conditions with the substrate 1c led to the formation of the diiodide 3c (Scheme 3). When a methoxy group is present on the phenyl ring, the ortho sites are acidified by its inductive effect and their pKa values become similar to those of the quinoline pyrido hydrogens. This could explain the formation of a heteroaryl compound metallated both at the 8 position peri to the quinoline nitrogen and at the 3′ position ortho to the methoxy group.
From the substrate 1d and the lithium–zinc base, the diiodide 3d was predominately formed (Scheme 4). This can be rationalized in terms of a huge pKa values difference in favour of benzylic hydrogens. pKa (THF) values less than 20 are typical of those for fluorenes with acceptor substituents.19 The same effect, enhanced by a larger number of potential deprotonation sites, was noted in the case of 1e, leading to a mixture of iodides.
In the case of the substrate 1f, with a 3-pyridyl group connected to the 2 position of the quinoline ring, deproto-metallation was only observed on the pyridine group in spite of higher acidities at the quinoline pyrido ring (Scheme 5). Such a result could be rationalized by a coordination exerted by the pyridine nitrogen toward a metal, either intermolecularly (approach of the metallic base) or intramolecularly (internal stabilization of the metallated species). The result suggests a more unlikely coordination by the quinoline nitrogen, maybe for steric reasons.
Upon treatment by the lithium–zinc and lithium–copper base, the quinolines 2-substituted by a 2-furyl and 2-thienyl group 1g,h were functionalized on the five-membered ring, and mainly at the 5′ position (Schemes 6, 7 and 9). The reason why there is no functionalization at the 8 position of the quinoline ring could be in relation with the presence of a clearly more acidic site at the position adjacent to the furan and thiophene heteroatom.
These results, together with earlier findings for azines7c and biaryls,10j lead us to the following conclusions. The pKa values obtained by our theoretical protocol are useful for the reaction outcome prediction. When using amido-based bimetallic bases in THF solution, it seems that there is a boundary corresponding to a pKa value of ca. 36. Consequently, the substrates with C–H bonds of enough acidity are deprotonated at the most acidic site in THF at moderate temperatures. The other option implies a substitution at a position adjacent to the ring nitrogen through metal complexation for weak CH acids (this course of the reaction could be enhanced by using less polar solvents at low temperatures).
Conclusions
Upon treatment with the lithium–zinc base prepared in situ from ZnCl2·TMEDA (0.5 equiv.) and LiTMP (1.5 equiv.), different 2-arylquinolines were deproto-metallated, as evidenced by subsequent interception by iodine. In the presence of unsubstituted phenyl and naphthyl substituents, a single reaction at the 8 position was noted; in contrast, with a 4-anisyl group bearing a substituent able to acidify the neighbouring hydrogens, a double functionalization took place at both the 8 and 3′ position. When a 3-pyridyl, a 2-furyl or a 2-thienyl was connected to the 2 position of the quinoline ring, the reaction occurred on this heteroaryl substituent, next to nitrogen, oxygen or sulfur, respectively. The iodo derivatives could be elaborated, for example by Suzuki cross-coupling as shown recently in the pyrazole series.10eUsing the corresponding lithiocuprate generated from CuCl (1 equiv.) and LiTMP (2 equiv.), a similar regioselectivity was observed. 2-Chlorophenyl ketones could be prepared, and a subsequent palladium-catalysed cyclization to polycycle demonstrated.
The CH acidities of the substrates in THF solution, which were calculated using a continuum solvation model, were used to rationalize the outcome of the reactions. The related substances could be preliminary functionalized to enhance the regioselectivity of deproto-metallation.
Experimental
General methods
Metallation reactions were performed under an argon atmosphere. THF was distilled over sodium/benzophenone. Column chromatography separations were achieved on silica gel (40–63 μm). Melting points were measured on a Kofler apparatus. IR spectra were taken on a Perkin-Elmer Spectrum 100 spectrometer. 1H and 13C Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker Avance III spectrometer at 300 and 75 MHz, respectively. 1H chemical shifts (δ) are given in ppm relative to the solvent residual peak, 13C chemical shifts are relative to the central peak of the solvent signal.27 Mass spectra (HRMS) measurements were performed at the CRMPO (Centre Régional de Mesures Physiques de l'Ouest) of Rennes using a Waters Q-TOF 2 instrument.General procedure 1 for the synthesis of the substituted quinolines 1a–h11
To 2-nitrobenzaldehyde (1.5 g, 10 mmol) in EtOH (20 mL) was added iron powder (2.2 g, 40 mmol) and 0.1 N aqueous HCl (5 mL, 0.5 mmol). The mixture was then stirred at 95 °C for 1 h. The required ketone (10 mmol) was then added. KOH (0.67 g, 12 mmol) was introduced portionwise (Caution! Potential exotherm) before stirring at 95 °C for 30 min. The mixture was cooled to room temperature, diluted with CH2Cl2 (300 mL) and filtered over silica. The filtrate was dried over MgSO4, and the solvents were removed under vacuum. The crude product was purified by chromatography over silica gel (eluent: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt).
1 heptane–AcOEt).
General procedure 2 for the deprotonative metallation using the lithium–zinc base followed by iodination
To a stirred, cooled (0 °C) solution of 2,2,6,6-tetramethylpiperidine (0.26 mL, 1.5 mmol) in THF (3 mL) was added BuLi (about 1.6 M hexanes solution, 1.5 mmol). After 15 min at 0 °C, ZnCl2·TMEDA (0.13 g, 0.50 mmol) was added, and the mixture was stirred for 15 min at this temperature before introduction of the substrate (1.0 mmol). After 2 h at room temperature, a solution of I2 (0.38 g, 1.5 mmol) in THF (5 mL) was added. The mixture was stirred overnight before addition of an aqueous saturated solution of Na2S2O3 (10 mL) and extraction with CH2Cl2 (3 × 20 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure before purification by flash chromatography on silica gel (the eluent is given in the product description).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 74% yield (0.25 g) as a yellow solid: mp 89 °C; IR (ATR): 2349, 1596, 1483, 1284, 954, 835, 760, 689 cm−1; 1H NMR (CDCl3, 300 MHz) 7.23 (dd, 1H, J = 7.4 and 8.0 Hz), 7.48–7.58 (m, 3H), 7.80 (dd, 1H, J = 1.3 and 8.0 Hz), 7.95 (d, 1H, J = 8.6 Hz), 8.13 (d, 1H, J = 8.6 Hz), 8.33–8.38 (m, 3H); 13C NMR (CDCl3, 75 MHz) 104.7, 119.2, 127.5, 127.7, 127.8, 128.4, 128.9, 129.0, 129.1, 129.9, 137.5, 138.7, 140.1, 146.7, 157.7.
1 heptane–AcOEt) in 74% yield (0.25 g) as a yellow solid: mp 89 °C; IR (ATR): 2349, 1596, 1483, 1284, 954, 835, 760, 689 cm−1; 1H NMR (CDCl3, 300 MHz) 7.23 (dd, 1H, J = 7.4 and 8.0 Hz), 7.48–7.58 (m, 3H), 7.80 (dd, 1H, J = 1.3 and 8.0 Hz), 7.95 (d, 1H, J = 8.6 Hz), 8.13 (d, 1H, J = 8.6 Hz), 8.33–8.38 (m, 3H); 13C NMR (CDCl3, 75 MHz) 104.7, 119.2, 127.5, 127.7, 127.8, 128.4, 128.9, 129.0, 129.1, 129.9, 137.5, 138.7, 140.1, 146.7, 157.7.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–CH2Cl2) in 54% yield (0.21 g) as a yellow solid: mp 210 °C; 1H NMR (CDCl3, 300 MHz) 7.19 (dd, 1H, J = 7.5 and 8.0 Hz), 7.53 (m, 2H), 7.74 (dd, 1H, J = 1.2 and 8.0 Hz), 7.88–8.09 (m, 5H), 8.34 (dd, 1H, J = 1.2 and 7.5 Hz), 8.60 (dd, 1H, J = 1.7 and 8.6 Hz), 8.68 (br s, 1H); 13C NMR (CDCl3, 75 MHz) 104.8, 119.3, 125.2, 126.4, 127.0, 127.3, 127.5, 127.7, 127.8, 128.4, 128.7, 129.0, 133.5, 134.2, 136.0, 137.5, 140.1, 146.8, 157.5; HRMS (ESI): calcd for C19H12IN [M + H]+ 381.0014, found 381.0012.
1 heptane–CH2Cl2) in 54% yield (0.21 g) as a yellow solid: mp 210 °C; 1H NMR (CDCl3, 300 MHz) 7.19 (dd, 1H, J = 7.5 and 8.0 Hz), 7.53 (m, 2H), 7.74 (dd, 1H, J = 1.2 and 8.0 Hz), 7.88–8.09 (m, 5H), 8.34 (dd, 1H, J = 1.2 and 7.5 Hz), 8.60 (dd, 1H, J = 1.7 and 8.6 Hz), 8.68 (br s, 1H); 13C NMR (CDCl3, 75 MHz) 104.8, 119.3, 125.2, 126.4, 127.0, 127.3, 127.5, 127.7, 127.8, 128.4, 128.7, 129.0, 133.5, 134.2, 136.0, 137.5, 140.1, 146.8, 157.5; HRMS (ESI): calcd for C19H12IN [M + H]+ 381.0014, found 381.0012.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 61% yield (0.30 g) as a yellow solid: mp 158 °C; 1H NMR (CDCl3, 300 MHz) 3.97 (s, 3H), 6.98 (d, 1H, J = 8.7 Hz), 7.22 (dd, 1H, J = 7.5 and 8.0 Hz), 7.78 (dd, 1H, J = 1.2 and 8.0 Hz), 7.86 (d, 1H, J = 8.6 Hz), 8.1 (d, 1H, J = 8.6 Hz), 8.33 (dd, 1H, J = 1.2 and 7.5 Hz), 8.37 (dd, 1H, J = 2.2 and 8.7 Hz), 8.74 (d, 1H, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) 56.7, 86.5, 104.5, 111.0, 118.6, 127.5, 127.6, 128.4, 129.3, 133.2, 137.7, 138.9, 140.3, 146.7, 155.9, 159.7; HRMS (ESI): calcd for C16H11I2NO [M + H]+ 486.8930, found 486.8927.
1 heptane–AcOEt) in 61% yield (0.30 g) as a yellow solid: mp 158 °C; 1H NMR (CDCl3, 300 MHz) 3.97 (s, 3H), 6.98 (d, 1H, J = 8.7 Hz), 7.22 (dd, 1H, J = 7.5 and 8.0 Hz), 7.78 (dd, 1H, J = 1.2 and 8.0 Hz), 7.86 (d, 1H, J = 8.6 Hz), 8.1 (d, 1H, J = 8.6 Hz), 8.33 (dd, 1H, J = 1.2 and 7.5 Hz), 8.37 (dd, 1H, J = 2.2 and 8.7 Hz), 8.74 (d, 1H, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) 56.7, 86.5, 104.5, 111.0, 118.6, 127.5, 127.6, 128.4, 129.3, 133.2, 137.7, 138.9, 140.3, 146.7, 155.9, 159.7; HRMS (ESI): calcd for C16H11I2NO [M + H]+ 486.8930, found 486.8927.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 4% yield (14 mg) as a yellow solid: mp 211 °C (rapidly decomposes by loss of iodine); IR (ATR): 3742, 3417, 3238, 2925, 2329, 1717, 1621, 1459, 1380, 1257, 1175, 1138, 856, 735 cm−1; 1H NMR (CDCl3, 300 MHz) 5.51 (s, 1H), 7.48–7.55 (m, 3H), 7.71 (ddd, 1H, J = 1.4, 6.9 and 8.4), 7.81 (m, 1H), 7.88 (dd, 1H, J = 1.2 and 8.1), 8.23 (br d, 1H, J = 8.4), 8.33 (m, 2H).
1 heptane–AcOEt) in 4% yield (14 mg) as a yellow solid: mp 211 °C (rapidly decomposes by loss of iodine); IR (ATR): 3742, 3417, 3238, 2925, 2329, 1717, 1621, 1459, 1380, 1257, 1175, 1138, 856, 735 cm−1; 1H NMR (CDCl3, 300 MHz) 5.51 (s, 1H), 7.48–7.55 (m, 3H), 7.71 (ddd, 1H, J = 1.4, 6.9 and 8.4), 7.81 (m, 1H), 7.88 (dd, 1H, J = 1.2 and 8.1), 8.23 (br d, 1H, J = 8.4), 8.33 (m, 2H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 51% yield (0.24 g) as an orange liquid: IR (ATR): 3333, 2933, 2345, 1713, 1621, 1604, 1578, 1387, 1039, 924, 864, 766 cm−1; 1H NMR (CDCl3, 300 MHz) 7.52–7.91 (m, 6H), 8.12 (m, 1H), 8.39 (m, 2H); 13C NMR (CDCl3, 75 MHz) 26.2, 122.0, 124.3, 126.6, 127.4, 128.3, 130.0, 130.4, 130.7, 131.8, 132.3, 132.7, 135.8, 137.6, 144.0, 150.8; HRMS (ESI): calcd for C16H9I2N [M + H]+ 468.8824, found 468.8820.
1 heptane–AcOEt) in 51% yield (0.24 g) as an orange liquid: IR (ATR): 3333, 2933, 2345, 1713, 1621, 1604, 1578, 1387, 1039, 924, 864, 766 cm−1; 1H NMR (CDCl3, 300 MHz) 7.52–7.91 (m, 6H), 8.12 (m, 1H), 8.39 (m, 2H); 13C NMR (CDCl3, 75 MHz) 26.2, 122.0, 124.3, 126.6, 127.4, 128.3, 130.0, 130.4, 130.7, 131.8, 132.3, 132.7, 135.8, 137.6, 144.0, 150.8; HRMS (ESI): calcd for C16H9I2N [M + H]+ 468.8824, found 468.8820.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 47% yield (0.16 g) as a yellow solid: mp 151 °C; IR (ATR): 2925, 1737, 1420, 1380, 1015, 793, 757 cm−1; 1H NMR (CDCl3, 300 MHz) 7.57 (ddd, 1H, J = 0.9, 7.0 and 7.9 Hz), 7.76 (ddd, 1H, J = 1.3, 7.0 and 8.4 Hz), 7.85 (m, 3H), 8.16 (m, 2H), 8.26 (d, 1H, J = 8.6 Hz), 9.07 (d, 1H, J = 2.5 Hz); 13C NMR (CDCl3, 75 MHz) 118.3, 119.2, 127.3, 127.6, 127.7, 129.6, 130.5, 134.5, 135.2, 136.8, 137.8, 148.2, 149.7, 153.5; HRMS (ESI): calcd for C14H9IN2 [M + H]+ 331.9810, found 331.9804.
1 heptane–AcOEt) in 47% yield (0.16 g) as a yellow solid: mp 151 °C; IR (ATR): 2925, 1737, 1420, 1380, 1015, 793, 757 cm−1; 1H NMR (CDCl3, 300 MHz) 7.57 (ddd, 1H, J = 0.9, 7.0 and 7.9 Hz), 7.76 (ddd, 1H, J = 1.3, 7.0 and 8.4 Hz), 7.85 (m, 3H), 8.16 (m, 2H), 8.26 (d, 1H, J = 8.6 Hz), 9.07 (d, 1H, J = 2.5 Hz); 13C NMR (CDCl3, 75 MHz) 118.3, 119.2, 127.3, 127.6, 127.7, 129.6, 130.5, 134.5, 135.2, 136.8, 137.8, 148.2, 149.7, 153.5; HRMS (ESI): calcd for C14H9IN2 [M + H]+ 331.9810, found 331.9804.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 20% yield (66 mg) as a yellow solid: mp 108 °C; IR (ATR): 3039, 2922, 2849, 1740, 1594, 1570, 1555, 1548, 1501, 1421, 1380, 1046, 1025, 834, 802, 728 cm−1; 1H NMR (CDCl3, 300 MHz) 7.40 (dd, 1H, J = 4.7 and 7.6 Hz), 7.62 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.70 (d, 1H, J = 8.5 Hz), 7.78 (ddd, 1H, J = 1.5, 6.9 and 8.3 Hz), 7.80 (dd, 1H, J = 2.0 and 7.6 Hz), 7.90 (dd, 1H, J = 1.2 and 8.1 Hz), 8.16 (br d, 1H, J = 8.3 Hz), 8.27 (br d, 1H, J = 8.5 Hz), 8.43 (dd, 1H, J = 2.0 and 4.7 Hz); 13C NMR (CDCl3, 75 MHz) 120.3, 122.4, 123.1, 127.3, 127.4, 127.8, 129.7, 130.3, 136.4, 137.8, 143.1, 147.9, 150.3, 158.6; HRMS (ESI): calcd for C14H9IN2 [M + H]+ 331.9810, found 331.9802.
1 heptane–AcOEt) in 20% yield (66 mg) as a yellow solid: mp 108 °C; IR (ATR): 3039, 2922, 2849, 1740, 1594, 1570, 1555, 1548, 1501, 1421, 1380, 1046, 1025, 834, 802, 728 cm−1; 1H NMR (CDCl3, 300 MHz) 7.40 (dd, 1H, J = 4.7 and 7.6 Hz), 7.62 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.70 (d, 1H, J = 8.5 Hz), 7.78 (ddd, 1H, J = 1.5, 6.9 and 8.3 Hz), 7.80 (dd, 1H, J = 2.0 and 7.6 Hz), 7.90 (dd, 1H, J = 1.2 and 8.1 Hz), 8.16 (br d, 1H, J = 8.3 Hz), 8.27 (br d, 1H, J = 8.5 Hz), 8.43 (dd, 1H, J = 2.0 and 4.7 Hz); 13C NMR (CDCl3, 75 MHz) 120.3, 122.4, 123.1, 127.3, 127.4, 127.8, 129.7, 130.3, 136.4, 137.8, 143.1, 147.9, 150.3, 158.6; HRMS (ESI): calcd for C14H9IN2 [M + H]+ 331.9810, found 331.9802.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 71% yield (0.23 g) as a brown solid: mp 159 °C; IR (ATR): 1595, 1500, 1066, 1013, 780 cm−1; 1H NMR (CDCl3, 300 MHz) 6.75 (d, 1H, J = 3.4 Hz), 7.17 (d, 1H, J = 3.4 Hz), 7.50 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.70 (ddd, 1H, J = 1.3, 6.9 and 8.7 Hz), 7.78 (dd, 1H, J = 1.3 and 8.1 Hz), 7.82 (d, 1H, J = 8.6 Hz), 8.09 (br d, 1H, J = 8.7 Hz), 8.16 (br d, 1H, J = 8.6 Hz); 13C NMR (CDCl3, 75 MHz) 90.5, 112.8, 117.3, 123.2, 126.6, 127.4, 127.7, 129.2, 130.2, 137.1, 147.8, 147.9, 159.0; HRMS (ESI): calcd for C13H8INO [M + H]+ 320.9651, found 320.9652.
1 heptane–AcOEt) in 71% yield (0.23 g) as a brown solid: mp 159 °C; IR (ATR): 1595, 1500, 1066, 1013, 780 cm−1; 1H NMR (CDCl3, 300 MHz) 6.75 (d, 1H, J = 3.4 Hz), 7.17 (d, 1H, J = 3.4 Hz), 7.50 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.70 (ddd, 1H, J = 1.3, 6.9 and 8.7 Hz), 7.78 (dd, 1H, J = 1.3 and 8.1 Hz), 7.82 (d, 1H, J = 8.6 Hz), 8.09 (br d, 1H, J = 8.7 Hz), 8.16 (br d, 1H, J = 8.6 Hz); 13C NMR (CDCl3, 75 MHz) 90.5, 112.8, 117.3, 123.2, 126.6, 127.4, 127.7, 129.2, 130.2, 137.1, 147.8, 147.9, 159.0; HRMS (ESI): calcd for C13H8INO [M + H]+ 320.9651, found 320.9652.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 heptane–CH2Cl2) in 52% yield (0.18 g) as a yellow solid: mp 156 °C; IR (ATR): 1595, 1499, 1418, 819, 782 cm−1; 1H NMR (CDCl3, 300 MHz) 7.31 (d, 1H, J = 3.9 Hz), 7.38 (d, 1H, J = 3.9 Hz), 7.50 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.71 (ddd, 1H, J = 1.5, 6.9 and 8.4 Hz), 7.72 (d, 1H, J = 8.6 Hz), 7.78 (br d, 1H, J = 8.1 Hz), 8.07 (br d, 1H, J = 8.4 Hz), 8.15 (br d, 1H, J = 8.6 Hz); 13C NMR (CDCl3, 75 MHz) 78.3, 117.0, 126.4, 126.9, 127.3, 127.6, 129.2, 130.1, 136.9, 138.1, 148.0, 151.3, 151.3.
2 heptane–CH2Cl2) in 52% yield (0.18 g) as a yellow solid: mp 156 °C; IR (ATR): 1595, 1499, 1418, 819, 782 cm−1; 1H NMR (CDCl3, 300 MHz) 7.31 (d, 1H, J = 3.9 Hz), 7.38 (d, 1H, J = 3.9 Hz), 7.50 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.71 (ddd, 1H, J = 1.5, 6.9 and 8.4 Hz), 7.72 (d, 1H, J = 8.6 Hz), 7.78 (br d, 1H, J = 8.1 Hz), 8.07 (br d, 1H, J = 8.4 Hz), 8.15 (br d, 1H, J = 8.6 Hz); 13C NMR (CDCl3, 75 MHz) 78.3, 117.0, 126.4, 126.9, 127.3, 127.6, 129.2, 130.1, 136.9, 138.1, 148.0, 151.3, 151.3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 heptane–CH2Cl2) in 12% yield (56 mg) as a yellow solid: mp 156 °C; IR (ATR): 1590, 1493, 1422, 1321, 949, 785, 757 cm−1; 1H NMR (CDCl3, 300 MHz) 7.35 (s, 1H), 7.55 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.73 (ddd, 1H, J = 1.3, 6.9 and 8.5 Hz), 7.82 (dd, 1H, J = 1.3 and 8.1 Hz), 8.10 (br d, 1H, J = 8.5 Hz), 8.23 (br d, 1H, J = 8.7 Hz), 8.29 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 79.6, 103.5, 117.5, 127.2, 127.5, 127.7, 128.4, 137.6, 138.1, 140.5, 146.7, 151.1, 152.1.
2 heptane–CH2Cl2) in 12% yield (56 mg) as a yellow solid: mp 156 °C; IR (ATR): 1590, 1493, 1422, 1321, 949, 785, 757 cm−1; 1H NMR (CDCl3, 300 MHz) 7.35 (s, 1H), 7.55 (ddd, 1H, J = 1.2, 6.9 and 8.1 Hz), 7.73 (ddd, 1H, J = 1.3, 6.9 and 8.5 Hz), 7.82 (dd, 1H, J = 1.3 and 8.1 Hz), 8.10 (br d, 1H, J = 8.5 Hz), 8.23 (br d, 1H, J = 8.7 Hz), 8.29 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 79.6, 103.5, 117.5, 127.2, 127.5, 127.7, 128.4, 137.6, 138.1, 140.5, 146.7, 151.1, 152.1.General procedure 3 for the deprotonative metallation using the lithium–copper base followed by aroylation
A stirred cooled (0 °C) solution of LiTMP prepared at 0 °C in THF (3 mL) from 2,2,6,6-tetramethylpiperidine (0.34 mL, 2.0 mmol) and BuLi (1.6 M hexanes solution, 2.0 mmol) was treated with TMEDA (0.15 mL, 1.0 mmol) and CuCl (99 mg, 1.0 mmol). The mixture was stirred for 15 min at 0 °C before introduction of the required substrate (1.0 mmol). After 2 h at rt, a solution of 2-chlorobenzoyl chloride (0.25 mL, 2.0 mmol) in THF (3 mL) was added. The mixture was stirred at 60 °C overnight before addition of a 1 M aqueous solution of NaOH (10 mL) and extraction with Et2O (2 × 20 mL). After washing the organic phase with an aqueous saturated solution of NH4Cl (10 mL) and drying over anhydrous Na2SO4, the solvent was evaporated under reduced pressure, and the product was isolated after purification by flash chromatography on silica gel (the eluent is given in the product description).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 58% yield (0.20 g) as a yellow powder; mp 126 °C; IR (ATR): 3627, 3496, 3056, 2607, 1665, 1584, 1544, 1437, 1344, 1288, 1239, 1074, 1033, 817, 751, 700, 688 cm−1; 1H NMR (CDCl3, 300 MHz) 7.15–7.32 (m, 6H), 7.42 (dd, 2H, J = 1.5 and 8.2 Hz), 7.52 (dd, 1H, J = 7.3 and 8.0 Hz), 7.57–7.60 (m, 1H), 7.76 (d, 1H, J = 8.7 Hz), 7.88 (dd, 1H, J = 1.4 and 8.1 Hz), 8.07 (dd, 1H, J = 1.5 and 7.2 Hz) 8.08 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 118.6 (CH), 126.1 (CH), 126.8 (CH), 127.0 (C), 127.4 (2CH), 128.5 (2CH), 129.7 (CH), 130.4 (CH), 130.7 (CH), 131.3 (CH), 131.8 (CH), 131.9 (CH), 132.1 (C), 136.9 (CH), 138.3 (C), 138.5 (C), 141.5 (C), 145.9 (C), 156.4 (C), 197.2 (C); HRMS (ASAP): calcd for C22H1535ClNO [M + H]+ 344.0842, found 344.0842.
1 heptane–AcOEt) in 58% yield (0.20 g) as a yellow powder; mp 126 °C; IR (ATR): 3627, 3496, 3056, 2607, 1665, 1584, 1544, 1437, 1344, 1288, 1239, 1074, 1033, 817, 751, 700, 688 cm−1; 1H NMR (CDCl3, 300 MHz) 7.15–7.32 (m, 6H), 7.42 (dd, 2H, J = 1.5 and 8.2 Hz), 7.52 (dd, 1H, J = 7.3 and 8.0 Hz), 7.57–7.60 (m, 1H), 7.76 (d, 1H, J = 8.7 Hz), 7.88 (dd, 1H, J = 1.4 and 8.1 Hz), 8.07 (dd, 1H, J = 1.5 and 7.2 Hz) 8.08 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 118.6 (CH), 126.1 (CH), 126.8 (CH), 127.0 (C), 127.4 (2CH), 128.5 (2CH), 129.7 (CH), 130.4 (CH), 130.7 (CH), 131.3 (CH), 131.8 (CH), 131.9 (CH), 132.1 (C), 136.9 (CH), 138.3 (C), 138.5 (C), 141.5 (C), 145.9 (C), 156.4 (C), 197.2 (C); HRMS (ASAP): calcd for C22H1535ClNO [M + H]+ 344.0842, found 344.0842.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 30% yield (0.12 g) as a yellow powder: mp 192 °C; IR (ATR): 3050, 2345, 1673, 1590, 1558, 1284, 1265, 1036, 933, 827, 809, 755, 744, 727 cm−1; 1H NMR (CDCl3, 300 MHz) 7.40–7.53 (m, 5H), 7.60–7.75 (m, 4H), 7.81–7.87 (m, 2H), 8.02–8.06 (m, 2H), 8.14 (s, 1H), 8.67 (dd, 1H, J = 1.5 and 7.2 Hz), 8.26 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 118.9 (CH), 124.8 (CH), 126.2 (CH), 126.3 (CH), 126.8 (CH), 126.9 (CH), 127.0 (CH), 127.7 (CH), 128.1 (CH), 128.9 (CH), 130.5 (CH), 130.8 (CH), 131.5 (CH), 131.8 (CH), 131.9 (CH), 132.2 (C), 133.4 (C), 134.1 (C), 136.0 (C), 136.9 (CH), 137.9 (C), 138.4 (C), 141.5 (C), 146.1 (C), 156.3 (C), 197.3 (C); HRMS (ESI): calcd for C26H1735ClNO [M + H]+ 394.0999, found 394.1009.
1 heptane–AcOEt) in 30% yield (0.12 g) as a yellow powder: mp 192 °C; IR (ATR): 3050, 2345, 1673, 1590, 1558, 1284, 1265, 1036, 933, 827, 809, 755, 744, 727 cm−1; 1H NMR (CDCl3, 300 MHz) 7.40–7.53 (m, 5H), 7.60–7.75 (m, 4H), 7.81–7.87 (m, 2H), 8.02–8.06 (m, 2H), 8.14 (s, 1H), 8.67 (dd, 1H, J = 1.5 and 7.2 Hz), 8.26 (d, 1H, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz) 118.9 (CH), 124.8 (CH), 126.2 (CH), 126.3 (CH), 126.8 (CH), 126.9 (CH), 127.0 (CH), 127.7 (CH), 128.1 (CH), 128.9 (CH), 130.5 (CH), 130.8 (CH), 131.5 (CH), 131.8 (CH), 131.9 (CH), 132.2 (C), 133.4 (C), 134.1 (C), 136.0 (C), 136.9 (CH), 137.9 (C), 138.4 (C), 141.5 (C), 146.1 (C), 156.3 (C), 197.3 (C); HRMS (ESI): calcd for C26H1735ClNO [M + H]+ 394.0999, found 394.1009.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 62% yield as a yellow powder: mp 160 °C; IR (ATR): 3750, 3060, 2329, 1646, 1592, 1471, 1426, 1300, 1263, 1237, 1049, 854, 820, 761, 749, 739, 696 cm−1; 1H NMR (CDCl3, 300 MHz) 7.25–7.45 (m, 6H), 7.59–7.71 (m, 4H), 7.8 (d, 1H, J = 8.4 Hz), 8.1 (d, 1H, J = 8.5 Hz); 13C NMR (CDCl3, 75 MHz) 117.8 (CH), 126.3 (CH), 126.7 (CH), 127.1 (CH), 127.7 (CH), 127.8 (C), 129.0 (CH), 129.6 (CH), 130.3 (CH), 130.4 (C), 131.3 (CH), 131.4 (CH), 136.4 (CH), 137.1 (CH), 138.4 (C), 144.5 (C), 148.1 (C), 151.0 (C), 154.5 (C), 187.3 (C); HRMS (ESI): calcd for C20H1235ClNOS [M + H]+ 350.0406, found 350.0411.
1 heptane–AcOEt) in 62% yield as a yellow powder: mp 160 °C; IR (ATR): 3750, 3060, 2329, 1646, 1592, 1471, 1426, 1300, 1263, 1237, 1049, 854, 820, 761, 749, 739, 696 cm−1; 1H NMR (CDCl3, 300 MHz) 7.25–7.45 (m, 6H), 7.59–7.71 (m, 4H), 7.8 (d, 1H, J = 8.4 Hz), 8.1 (d, 1H, J = 8.5 Hz); 13C NMR (CDCl3, 75 MHz) 117.8 (CH), 126.3 (CH), 126.7 (CH), 127.1 (CH), 127.7 (CH), 127.8 (C), 129.0 (CH), 129.6 (CH), 130.3 (CH), 130.4 (C), 131.3 (CH), 131.4 (CH), 136.4 (CH), 137.1 (CH), 138.4 (C), 144.5 (C), 148.1 (C), 151.0 (C), 154.5 (C), 187.3 (C); HRMS (ESI): calcd for C20H1235ClNOS [M + H]+ 350.0406, found 350.0411.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane–AcOEt) in 45% yield (0.14 g) as a yellow powder; mp 164 °C; IR (ATR): 3623, 2921, 2345, 1704, 1601, 1547, 1462, 1312, 1278, 1175, 1152, 844, 758, 697 cm−1; 1H NMR (CDCl3, 300 MHz) 7.34 (1H, dt, J = 0.9 and 7.3 Hz), 7.45–7.59 (m, 5H), 7.69–7.73 (m, 2H), 7.89 (d, 1H, J = 8.7 Hz), 7.98 (d, 1H, J = 8.1 Hz), 8.15 (d, 1H, J = 8.7 Hz), 8.36–8.39 (m, 2H); 13C NMR (CDCl3, 75 MHz) 118.5 (CH), 118.7 (CH), 120.4 (CH), 124.1 (CH), 127.1 (C), 127.9 (2CH), 128.5 (C), 129.0 (2CH), 130.0 (CH), 130.3 (CH), 134.1 (CH), 134.7 (C), 135.5 (CH), 137.3 (CH), 138.8 (C), 143.1 (C), 144.9 (C), 149.7 (C), 159.9 (C), 192.8 (C); HRMS (ASAP): calcd for C22H14NO [M + H]+ 308.1075, found 308.1074.
1 heptane–AcOEt) in 45% yield (0.14 g) as a yellow powder; mp 164 °C; IR (ATR): 3623, 2921, 2345, 1704, 1601, 1547, 1462, 1312, 1278, 1175, 1152, 844, 758, 697 cm−1; 1H NMR (CDCl3, 300 MHz) 7.34 (1H, dt, J = 0.9 and 7.3 Hz), 7.45–7.59 (m, 5H), 7.69–7.73 (m, 2H), 7.89 (d, 1H, J = 8.7 Hz), 7.98 (d, 1H, J = 8.1 Hz), 8.15 (d, 1H, J = 8.7 Hz), 8.36–8.39 (m, 2H); 13C NMR (CDCl3, 75 MHz) 118.5 (CH), 118.7 (CH), 120.4 (CH), 124.1 (CH), 127.1 (C), 127.9 (2CH), 128.5 (C), 129.0 (2CH), 130.0 (CH), 130.3 (CH), 134.1 (CH), 134.7 (C), 135.5 (CH), 137.3 (CH), 138.8 (C), 143.1 (C), 144.9 (C), 149.7 (C), 159.9 (C), 192.8 (C); HRMS (ASAP): calcd for C22H14NO [M + H]+ 308.1075, found 308.1074.Crystallography
The samples were studied with graphite monochromatized Mo-Kα radiation (λ = 0.71073 Å). X-ray diffraction data were collected at T = 150(2) K using APEXII Bruker-AXS diffractometer. The structure was solved by direct methods using the SIR97 program,33 and then refined with full-matrix least-square methods based on F2 (SHELX-97)34 with the aid of the WINGX program.35 All non-hydrogen atoms were refined with anisotropic atomic displacement parameters. H atoms were finally included in their calculated positions. Molecular diagrams were generated by ORTEP-3 (version 2.02).Acknowledgements
We are grateful to the Agence Nationale de la Recherche for financial support to NM and GB. FM thanks the Institut Universitaire de France and Rennes Métropole for financial support. We also thank Thermo Fischer for generous gift of 2,2,6,6-tetramethylpiperidine.Notes and references
- T. Eicher, S. Hauptmann and A. Speicher, The Chemistry of Heterocycles, Wiley-VCH, New York, 2nd edn, 2003 Search PubMed
.
- J. A. Joule and K. Mills, Heterocyclic Chemistry, Wiley-Blackwell, Chichester, 5th edn, 2010 Search PubMed
.
-
(a) H. W. Gschwend and H. R. Rodriguez, Org. React., 1979, 26, 1–360 CAS
; (b) P. Beak and V. Snieckus, Acc. Chem. Res., 1982, 15, 306–312 CrossRef CAS
; (c) V. Snieckus, Chem. Rev., 1990, 90, 879–933 CrossRef CAS
; (d) T. G. Gant and A. I. Meyers, Tetrahedron, 1994, 50, 2297–2360 CrossRef CAS
; (e) M. Schlosser, Organometallics in Synthesis, ed. M. Schlosser, Wiley, 2nd edn, 2002, ch. I Search PubMed
.
-
(a) F. Mongin and G. Queguiner, Tetrahedron, 2001, 57, 4059–4090 CrossRef CAS
; (b) J.-M. L'Helgoual'ch, F. Chevallier and F. Mongin, Targets Heterocycl. Syst., 2007, 11, 155–180 CAS
; (c) F. Chevallier and F. Mongin, Top. Heterocycl. Chem., 2013, 31, 93–130 Search PubMed
.
-
(a) R. E. Mulvey, Organometallics, 2006, 25, 1060–1075 CrossRef CAS
; (b) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802–3824 CrossRef CAS PubMed
; (c) R. E. Mulvey, Acc. Chem. Res., 2009, 42, 743–755 CrossRef CAS PubMed
; (d) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794–9824 CrossRef CAS PubMed
; (e) F. Mongin and M. Uchiyama, Curr. Org. Chem., 2011, 15, 2340–2361 CrossRef CAS
; (f) F. Mongin and A. Harrison-Marchand, Chem. Rev., 2013, 113, 7563–7727 CrossRef CAS PubMed
; (g) R. E. Mulvey, Dalton Trans., 2013, 42, 6676–6693 RSC
.
- See for example:
(a) M. Jaric, B. A. Haag, A. Unsinn, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 5451–5455 CrossRef CAS PubMed
; (b) M. Jeganmohan and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 8520–8524 CrossRef CAS PubMed
; (c) C. J. Rohbogner, S. Wirth and P. Knochel, Org. Lett., 2010, 12, 1984–1987 CrossRef CAS PubMed
; (d) M. Jaric, B. A. Haag, S. M. Manolikakes and P. Knochel, Org. Lett., 2011, 13, 2306–2309 CrossRef CAS PubMed
; (e) S. M. Manolikakes, M. Jaric, K. Karaghiosoff and P. Knochel, Chem. Commun., 2013, 49, 2124–2126 RSC
; (f) C. Metallinos and K. Stromski, Top. Heterocycl. Chem., 2013, 31, 65–92 Search PubMed
.
-
(a) T. T. Nguyen, F. Chevallier, V. Jouikov and F. Mongin, Tetrahedron Lett., 2009, 50, 6787–6790 CrossRef CAS PubMed
; (b) T. T. Nguyen, N. Marquise, F. Chevallier and F. Mongin, Chem.–Eur. J., 2011, 17, 10405–10416 CrossRef CAS PubMed
; (c) K. Snégaroff, T. T. Nguyen, N. Marquise, Y. S. Halauko, P. J. Harford, T. Roisnel, V. E. Matulis, O. A. Ivashkevich, F. Chevallier, A. E. H. Wheatley, P. C. Gros and F. Mongin, Chem.–Eur. J., 2011, 17, 13284–13297 CrossRef PubMed
; (d) N. Marquise, P. J. Harford, F. Chevallier, T. Roisnel, A. E. H. Wheatley, P. C. Gros and F. Mongin, Tetrahedron Lett., 2013, 54, 3154–3157 CrossRef CAS PubMed
; (e) N. Marquise, P. J. Harford, F. Chevallier, T. Roisnel, V. Dorcet, A.-L. Gagez, S. Sablé, L. Picot, V. Thiéry, A. E. H. Wheatley, P. C. Gros and F. Mongin, Tetrahedron, 2013, 69, 10123–10133 CrossRef CAS PubMed
.
- J. M. L'Helgoual'ch, A. Seggio, F. Chevallier, M. Yonehara, E. Jeanneau, M. Uchiyama and F. Mongin, J. Org. Chem., 2008, 73, 177–183 CrossRef CAS PubMed
.
- P. García-Álvarez, R. E. Mulvey and J. A. Parkinson, Angew. Chem., Int. Ed., 2011, 50, 9668–9671 CrossRef PubMed
.
-
(a) A. Seggio, M.-I. Lannou, F. Chevallier, D. Nobuto, M. Uchiyama, S. Golhen, T. Roisnel and F. Mongin, Chem.–Eur. J., 2007, 13, 9982–9989 CrossRef CAS PubMed
; (b) A. Seggio, F. Chevallier, M. Vaultier and F. Mongin, J. Org. Chem., 2007, 72, 6602–6605 CrossRef CAS PubMed
; (c) G. Dayaker, A. Sreeshailam, F. Chevallier, T. Roisnel, P. Radha Krishna and F. Mongin, Chem. Commun., 2010, 46, 2862–2864 RSC
; (d) K. Snégaroff, S. Komagawa, F. Chevallier, P. C. Gros, S. Golhen, T. Roisnel, M. Uchiyama and F. Mongin, Chem.–Eur. J., 2010, 16, 8191–8201 CrossRef PubMed
; (e) F. Chevallier, Y. S. Halauko, C. Pecceu, I. F. Nassar, T. U. Dam, T. Roisnel, V. E. Matulis, O. A. Ivashkevich and F. Mongin, Org. Biomol. Chem., 2011, 9, 4671–4684 RSC
; (f) G. Dayaker, D. Tilly, F. Chevallier, G. Hilmersson, P. C. Gros and F. Mongin, Eur. J. Org. Chem., 2012, 6051–6057 CrossRef CAS
; (g) F. Chevallier, T. Blin, E. Nagaradja, F. Lassagne, T. Roisnel, Y. S. Halauko, V. E. Matulis, O. A. Ivashkevich and F. Mongin, Org. Biomol. Chem., 2012, 10, 4878–4885 RSC
; (h) A. Sreeshailam, G. Dayaker, D. V. Ramana, F. Chevallier, T. Roisnel, S. Komagawa, R. Takita, M. Uchiyama, P. Radha Krishna and F. Mongin, RSC Adv., 2012, 2, 7030–7032 RSC
; (i) D. Tilly, K. Snégaroff, G. Dayaker, F. Chevallier, P. C. Gros and F. Mongin, Tetrahedron, 2012, 68, 8761–8766 CrossRef CAS PubMed
; (j) R. R. Kadiyala, D. Tilly, E. Nagaradja, T. Roisnel, V. E. Matulis, O. A. Ivashkevich, Y. S. Halauko, F. Chevallier, P. C. Gros and F. Mongin, Chem.–Eur. J., 2013, 19, 7944–7960 CrossRef CAS PubMed
; (k) E. Nagaradja, F. Chevallier, T. Roisnel, V. Dorcet, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis and F. Mongin, Org. Biomol. Chem., 2014, 12, 1475–1487 RSC
.
- A.-H. Li, E. Ahmed, X. Chen, M. Cox, A. P. Crew, H.-Q. Dong, M. Jin, L. Ma, B. Panicker, K. W. Siu, A. G. Steinig, K. M. Stolz, P. A. R. Tavares, B. Volk, Q. Weng, D. Werner and M. J. Mulvihill, Org. Biomol. Chem., 2007, 5, 61–64 CAS
.
- N. Boudet, J. R. Lachs and P. Knochel, Org. Lett., 2007, 9, 5525–5528 CrossRef CAS PubMed
.
- Y. Kondo, M. Shilai, M. Uchiyama and T. Sakamoto, J. Am. Chem. Soc., 1999, 121, 3539–3540 CrossRef CAS
.
- X. Qu, Q. Liu, X. Ji, H. Chen, Z. Zhou and Z. Shen, Chem. Commun., 2012, 48, 4600–4602 RSC
.
-
(a) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS PubMed
; (b) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731–1770 CrossRef CAS PubMed
; (c) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed
.
-
(a) L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581–590 CrossRef CAS PubMed
; (b) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed
; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed
; (d) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed
.
- M. R. Netherton and G. C. Fu, Org. Lett., 2001, 3, 4295–4298 CrossRef CAS PubMed
.
- R. R. Fraser, T. S. Mansour and S. Savard, J. Org. Chem., 1985, 50, 3232–3234 CrossRef CAS
.
- A. Kütt, I. Leito, I. Kaljurand, L. Sooväli, V. M. Vlasov, L. M. Yagupolskii and I. A. Koppel, J. Org. Chem., 2006, 71, 2829–2838 CrossRef PubMed
.
- M. Meot-Ner, J. F. Liebman and S. A. Kafafi, J. Am. Chem. Soc., 1988, 110, 5937–5941 CrossRef CAS PubMed
.
- J. C. Del Valle and J. L. G. De Paz, J. Mol. Struct.: THEOCHEM, 1992, 86, 481–491 CrossRef CAS
.
- K. Shen, Y. Fu, J.-N. Li, L. Liu and Q.-X. Guo, Tetrahedron, 2007, 63, 1568–1576 CrossRef CAS PubMed
.
- S. Kheirjou, A. Abedin, A. Fattahi and M. M. Hashemi, Comput. Theor. Chem., 2014, 1027, 191–196 CrossRef CAS PubMed
.
- V. E. Matulis, Y. S. Halauko, O. A. Ivashkevich and P. N. Gaponik, J. Mol. Struct.: THEOCHEM, 2009, 909, 19–24 CrossRef CAS PubMed
.
- E. Cances, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041 CrossRef CAS PubMed
.
- S. Konishi, S. Kawamorita, T. Iwai, P. G. Steel, T. B. Marder and M. Sawamura, Chem.–Asian J., 2014, 9, 434–438 CrossRef CAS
.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS
.
- A. M. Berman, J. C. Lewis, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 14926–14927 CrossRef CAS PubMed
.
- R.-G. Xing, Y.-N. Li, Q. Liu, Y.-F. Han, X. Wei, J. Li and B. Zhou, Synthesis, 2011, 2066–2072 CAS
.
- A.-H. Li, D. J. Beard, H. Coate, A. Honda, M. Kadalbajoo, A. Kleinberg, R. Laufer, K. M. Mulvihill, A. Nigro, P. Rastogi, D. Sherman, K. W. Siu, A. G. Steinig, T. Wang, D. Werner, A. P. Crew and M. J. Mulvihill, Synthesis, 2010, 1678–1686 CrossRef CAS PubMed
.
- A. Canas-Rodriguez, R. G. Canas and A. Mateo-Bernardo, An. Quim., Ser. C, 1987, 83, 24–27 CAS
.
- R. Martinez, D. J. Ramon and M. Yus, Tetrahedron, 2006, 62, 8988–9001 CrossRef CAS PubMed
.
- A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori and R. Spagna, J. Appl. Crystallogr., 1999, 32, 115–119 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, calculated values of the Gibbs energies ΔacidG [kcal mol−1] for deprotonation at the corresponding positions of the investigated quinolines, and cartesian coordinates of molecular geometry for the most stable rotamer form of selected quinolines (on example of 1f, 1h) (neutral molecule, gas phase) optimized at B3LYP/6-31G(d) level of theory. CCDC 981282–981288. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02583k |
| ‡ CIF files available in the ESI: CIF files of 1e (CCDC 981282), 2a (981283), 2h (981284), 3h (981285), 4a (981286), 4b (981287), and 5a (981288). |
| This journal is © The Royal Society of Chemistry 2014 |