Carbon nanospheres grown on graphene as anodes for Li-ion batteries
Abstract
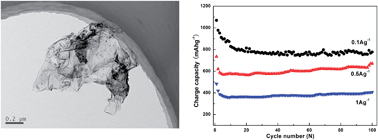
An electrode composed of graphene/carbon nanospheres is synthesized via in situ polymerization and carbonization, for Li-ion batteries. Carbon nanospheres derived from carbonized polypyrrole are grown on the surface of graphene and assembled into a flake-like structure. The synergistic effect between carbon and graphene improves the capacity and rate capability of the electrode.

 Please wait while we load your content...
Please wait while we load your content...