Facile production of a large-area flexible TiO2/carbon cloth for dye removal†
Le Yanga,
Zonghan Honga,
Jun Wua and
Lian-Wen Zhu*b
aCollege of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, People's Republic of China
bSchool of Biology and Chemical Engineering, Jiaxing University, Jiaxing, Zhejiang 314001, China. E-mail: lwzhu@mail.zjxu.edu.cn
First published on 29th May 2014
Abstract
Through the precoating of TiO2 on carbon fiber sheet and the subsequent hydrothermal reaction of the coated species with NaOH and tetrabutyl titanate, we successfully demonstrated the engineering growth of TiO2 nanofibers on carbon fiber sheet. The resulting cloth exhibits excellent flexibility and enables robust, large-area (∼300 cm2) fabrication, representing a significant advantage over previous brittle, small area nanofibrous macroscopic structures. The adsorption and photodecomposition of Rhodamine B in water showed that the resulting cloth is very convenient for the purification of contaminated water owing to its combined adsorption and cleaning function. This work provides new insight into the construction of a large area, flexible and robust water purification membrane material.
Introduction
Due to the rapid population growth and mounting environmental crisis, water shortage has become one of the top issues and is probably expected to be the most important problem in the future.1 Therefore, the development of new and multifunctional water purification materials is becoming an important task for the materials community. To meet the demand of practical water purification, the essential features of next generation water purification materials should be high efficiency, high durability, easy recovery, low cost, and environment beneath.In the past decade, macroscopic structures consisting of nano-units (e.g., nanowires, nanotubes, nanosheets, etc.) have been demonstrated as promising candidates for catalysts,2,3 energy-harvesting systems,4–6 absorbents and filters for liquid filtration and separation.7,8 Because the macroscopic structures not only retain the remarkable properties of nano building blocks, but also exhibit new distinctive properties such as high porosity, good mechanical strength, light weight, and multifunctionality.9
Owing to their ideal morphology and mechanical strength, carbon nanomaterials (e.g., carbon nanofibers, carbon nanotubes, graphene, etc.) were firstly selected as building blocks to be assembled into macroscopic structures, including free-standing membrane,10,11 vertically aligned array,12,13 porous sponge,14,15 and strong aerogel.16–18 These carbon-based macroscopic structures can effectively remove various contamination from water through a simple adsorption or filtration process. However, energy consuming post-treatments (organic solvent cleaning, high temperature calcination, etc.) are needed to remove the anchored contamination from the carbon-based macroscopic structures for the aim of regeneration, which limited their widespread application. Comparing with carbon macroscopic structures, TiO2 macroscopic structures exhibit easy regeneration feature, since TiO2 nanounits can provide photogenerated charges to decompose the pollutants on their surface under the illumination of ultraviolet light.19 Recent reports have demonstrated the superiorities of TiO2 macroscopic structures for water purification.20–24 However, the reported TiO2 macroscopic structures are always brattle and the size is small, which are the main obstacles to their widespread practical applications.
Carbon fiber sheet (CFS) is a kind of robust multipurpose material with high mechanical strength, good temperature and corrosion resistance. Therefore, the growth of TiO2 nanofibers on CFSs may give rise to robust macroscopic nano-architectures, which may serve as a class of recoverable and durable adsorbent and photocatalytic materials as they will greatly facilitate the treatment of wastewater. Traditional powder photocatalysts need to be dispersed into the waste water and therefore it is impractical to use them for river purification since recovery is almost impossible and causes secondary pollution.25 In comparison, the modified CFSs can be immersed into wastewater and organic pollutants will be captured by the high porous structures formed by the overlapping and interpenetration of the nanofibers. After the irradiation of UV light, the adsorbed organic pollutants could be eliminated. Once the water is cleaned, the CFSs together with TiO2 nanofibers can be easily lifted from the water.
In this work, through the precoating of TiO2 on CFSs and the subsequent hydrothermal reaction of the coated species with NaOH and tetrabutyl titanate (TBT), we achieved a large area, flexible and robust TiO2 nanofiber macroscopic structure. The adsorption and photodecomposition of Rhodamine B (RhB) in water demonstrated the great convenience of the products in the purification of contaminated water.
Experimental
Materials and methods
CFSs were commercially available (purchased from Jiaxing Wanwei Textile Co., Ltd.). The other reagents were all purchased from Shanghai Chemical Co. and used as received.Growth of TiO2 nanofibers on the CFSs
Before use, CFS was cleaned with 1 M NaOH solution and distilled water and was dried in an oven at 90 °C. The clean CFS was immersed into 15 vol% tetrabutyl titanate–hexane solution for 5 min, and thus the surface of CFS was wetted by tetrabutyl titanate–hexane solution. When CFS was taken out, hexane would volatilize rapidly and only tetrabutyl titanate would be left on the textile. Then, the textile was exposed to water vapor to promote the hydrolysis of tetrabutyl titanate and the generation of TiO2. This wetting–hydrolysis process was repeated three times in order to increase the loading capacity of TiO2. After that, the textile coated by TiO2 was rolled up and placed into an autoclave filled with 10 M NaOH solution and 0.5 mL tetrabutyl titanate. The size of the textile is dependent on the volume of the autoclave. For a 100 mL autoclave, the texture can be several hundreds of square centimeters in size. For a 1 L autoclave, the textile can be several square meters. After heated at 200 °C for 48 hours, the autoclave was cooled down to room temperature and the textile was taken out, washed alternatively with 0.1 M HAc and distilled water for five times, and calcined at 500 °C under air atmosphere for 3 hours. It could be seen that the CFS had changed its color from the initial black to white, which suggested the presence of TiO2.Characterization
The loading capacity of TiO2 on the textile was determined by comparison of the initial weight and the final weight of the textile. The morphology observations of the textile and the nanofibers were carried out on a HITACHI S-4800 scanning electron microscope (SEM). X-ray diffraction (XRD) patterns of the textile was recorded on an X'Pert PRO SUPER rA rotation anode X-ray diffractometer with Ni-filtered Cu-Kα radiation (λ = 1.5418 Å). Diffuse reflectance spectra (DRS) and UV-vis absorption spectra were recorded on a Cary 5000 UV-vis-near-infrared spectrophotometer fitted with an integrating sphere. The samples for SEM, XRD, and DRS characterizations were the textile directly. The samples for TEM characterization were prepared as follows: TiO2 nanofibers were exfoliated from the textile by ultrasound, dispersed in absolute ethanol, and dropped onto a carbon film supported on a copper grid.Evaluation of photocatalytic activity of TiO2/carbon cloth
RhB is a chemically stable and poorly biodegradable dye contaminant in wastewater. Here we use its decomposition as a simulation to demonstrate the great advantages of the modified textile in the purification of the contaminated water. At first, we studied the robustness and reusability of the modified textile by repeated adsorption–lifting–irradiation experiments. A piece of the modified textile (dimensions: 2 cm × 4 cm) was immersed into 100 mL RhB solution (concentration: 9 mg L−1) for 10 minutes. The modified textile was lifted from the solution and irradiated by a 10 W UV lamp (wavelength: 254 nm, light intensity: 0.08 mW cm−1). During the irradiation, the modified textile was placed 10 cm away from the lamp, and its each side was irradiated 30 minutes. The existence of the RhB molecules on the surface of the modified textile was monitored by measuring the diffuse reflection spectrum. The above adsorption–lifting–irradiation process was repeated ten times. Subsequently, we investigated the photodegradation behavior of RhB under the continuous photocatalysis of the modified textile. During these studies, the modified textile was immersed in the solution all the time. The concentration of the RhB solution was monitored by detecting the absorbance at 550 nm per 20 min.Results and discussion
Characterization of TiO2 nanofibers on the CFSs
Scheme 1 describes the process of the engineering growth of TiO2 nanofibers on the CFSs. Firstly, a piece of CFSs is soaked in TBT–hexane solution for wetting. The sizes of the textile are unrestricted as long as it can be held by the reactor. When the textile is taken out, hexane volatilizes rapidly and only TBT molecules are left on the skeletons of the CFSs. Once the resulting textile was exposed to water vapor, TBT molecules hydrolyze immediately and yield TiO2 particles on the skeletons of the textile (Fig. S1c and d, ESI†). The wetting–hydrolysis process is repeated three times to increase the loading capacity of TiO2 on the textile (Fig. S1e and f, ESI†), and is essential for the engineering growth of nanofiber on CFS. Secondly, the resulting textile was allowed to react with 10 M NaOH solution and 0.5 mL TBT under hydrothermal condition for the engineering growth of sodium titanate nanofibers on CFSs. When TBT was absent from the hydrothermal reaction, a small amount of nanofibers can be grown on the textile. Sodium titanate nanofibers could be converted into TiO2 nanofibers through the post-treatment of acid washing and calcination. Consequently, the TiO2 nanofiber modified CFSs is successfully engineered.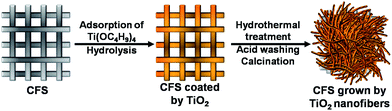 | ||
| Scheme 1 Schematic diagram showing the process for the engineering growth of TiO2 nanofibers on the CFSs. | ||
The hydrothermal reaction was accompanied by a color change from the initial black to white and resulted in the formation of Na2Ti3O7 nanofibers on CFSs,3 which is further confirmed by X-ray powder diffraction (XRD) test (Fig. 1, curve b). After annealed at 500 °C for 3 h, Na2Ti3O7 was transformed to TiO2. The XRD patterns of the as-prepared TiO2/carbon cloth are shown in Fig. 1, curve c. All the diffraction peaks can be indexed to TiO2 (JCPDs file, no. 35-0088), indicating the successful growth of TiO2 nanofibers on the textile. The XRD pattern of TiO2/carbon cloth showed a strong peak centered at 25.5°, demonstrating the good crystalline properties of TiO2 nanofibers. Notably, no typical diffraction peaks of the CFS are observed in the TiO2/carbon sample, which is attributed to the fact that the main peak of anatase TiO2 at 25.5° may be shielded the main characteristic peak of CFS.
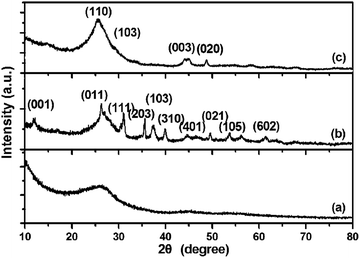 | ||
| Fig. 1 XRD patterns. (a) Pure CFS; (b) CFS grown by Na2Ti3O7 nanofibers; (c) CFS grown by TiO2 nanofibers. | ||
Fig. 2a and b show the digital photographs of CFSs and TiO2 nanofiber modified CFSs, respectively. White and uniform layers were formed and firmly attached on both sides of CFSs, indicating the successful growth of TiO2 nanofibers on CFS through the designed approach shown in Scheme 1. Furthermore, the size of the final products depends on the volume of the autoclave, since the present method is great versatile in scaling up the synthesis. A 150 mL autoclave can produce a textile with size of 300 cm2 (Fig. S2a, ESI†). For a 1 L autoclave, the size can be several square meters. So it is quite possible to realize larger scale synthesis for industrial production by further enlarging the equipment. As far as we know, it is difficult to achieve such large-size TiO2 nanofiber macroscopic structures with traditional methods.20,23,26,27 In addition, the CFS grown by inflexible TiO2 nanofibers is flexible and easily curled (Fig. S2b, ESI†), which is highly desirable for the construction of flexible devices, such as wearable energy harvesting and self-cleaning systems.
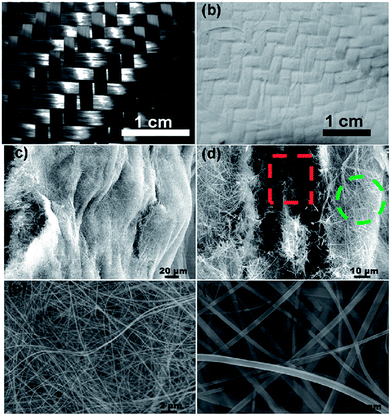 | ||
| Fig. 2 (a) Digital photograph of the CFSs; (b) digital photograph of the TiO2 nanofiber modified CFSs; (c–f) SEM images of the TiO2 nanofiber modified CFSs. | ||
A low-magnification SEM image (Fig. 2c) reveals that the white layers on both sides of CFS were consisting of long and flexible TiO2 nanofibers and the nanofibers are uniform, long, and ribbon-like and the diameters of the nanofibers are below 200 nm and longitudinal dimensions are several tens to several hundreds of micrometers. Further SEM observations (Fig. 2d) indicate that there are two kinds of nanofibers in the TiO2 nanofiber layers. One kind of nanofibers was formed on the surface of carbon fibres (indicated by green cycle in Fig. 2d), while another kind was formed between carbon fibres (indicated by red box in Fig. 2d). The nanofibers on the surface of the CFS were the hydrothermal reaction products of the added TBT with NaOH, while the nanofibers between carbon fibres were formed from the precoated TiO2 particles and their engineering growth was directed by the capillary forces between carbon fibers.
Both the precoated TiO2 particles and TBT are essential for the engineering growth of nanofibers on CFS. In the hydrothermal treatment process, the absorbed TiO2 particles on the surface of carbon fibers were transformed to long nanofibers and the as-prepared nanofibers were in situ anchored with CFS via the overlapping and interpenetration of the flexible TiO2 nanofibers and carbon fibres. When no TiO2 particles were precoated on CFS, few nanofibers were randomly introduced on the CFS, which can be easily washed off from the CFS because of the weak interaction between TiO2 and carbon fibers. The nanofibers obtained via the hydrothermal reaction of TBT and NaOH further interweaved with the nanofibers grown on CFS, leading to the formation of dense nanofiber layers on CFS. The layer thickness can be easily controlled via the amount of the TBT added. When TBT is absent from the hydrothermal reaction, only a small amount of nanofibers were formed on the CFS. 0.1 mL TBT gave rise to thin nanofiber layers, while 0.5 mL TBT leaded to the formation of thick layers (Fig. S3, ESI†). When more than 0.5 mL TBT was added to the reaction system, large amount of white precipitate was formed at the bottom of the autoclave, indicating the maximum amount of TiO2 nanofibers have been achieved on the CFS. Such a textile with a size of 4 cm2 can support 100 mg of TiO2 nanofibers.
Previously, coating of TiO2 onto polymers, cotton textiles and foams have been achieved by the method of impregnation or plasma sputtering, where TiO2 is composed of irregular nanoparticles.28–32 Comparing with nanoparticles, nanofibers permit easy electron transport in one dimension,33–35 which is favorable towards improving the photocatalytic performance. In addition, TiO2 nanoparticles supported by polymers or cotton textiles are not suitable for durable photocatalytic materials because the polymers and textiles may also be decomposed by TiO2. Furthermore, because of the weak interactions between TiO2 and substrate, the TiO2 may fall off the support during the photocatalytic process. In our case, TiO2 nanofibers were firmly anchored with the CFS not only via the overlapping and interpenetration, but also benefitting from the pre-coating process, in which the seed layers of TiO2 were firmly anchored on CFS. Since the pre-coating seeds were anchored on the CFS, the nanofibers growing out of them or on them are in situ firmly anchored on CFS. Consequently, the as-prepared textile can serve as a class of recoverable and durable photocatalytic materials without catalyst loss.
Optical properties
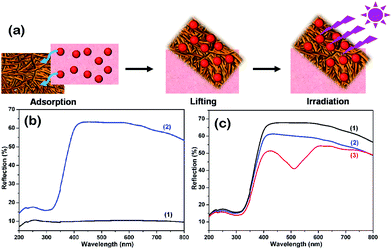
Fig. 3b shows the surface diffuse reflection spectra of the pure textile and the CFSs grown by the TiO2 nanofibers. Pure textile does not have characteristic absorption bands. But the modified textile exhibits an intense absorption towards light below 400 nm. Consequently, the modified textile can utilize light efficiently and provide photogenerated charges for photocatalytic reactions.Photocatalytic performance
As a chemically stable and poorly biodegradable dye, RhB is a main contaminant in wastewater. In this work, we use the photodegradation of RhB as a model reaction to evaluate the great advantages of the textile modified by TiO2 nanowires in the purification of polluted water. Firstly, we investigated the robustness and reusability of the prepared textile by repeated adsorption–lifting–irradiation experiments. As shown in Fig. 3a, the modified textile was immersed into the wastewater to capture RhB. Then the CFSs together with the TiO2 nanofibers and RhB was lifted from the water. Finally the textile was irradiated to remove RhB. Fig. 3c shows the diffuse reflection spectra of the modified textile at the three stages. Initially, only a sharp band around 400 nm was found in Fig. 3c(1), which is attributed to the TiO2 nanofibers on the textile. At the second stage, an additional band around 550 nm appeared in Fig. 3c(3), which is due to the chromophores in the RhB molecules. These results revealed that the RhB in the solution was captured by the modified textile. The quantity of the adsorbed RhB was calculated by measuring the concentration of the RhB solution before and after adsorption and the RhB adsorption capacity of the modified textile is 0.4 mg g−1 (Fig. S4, ESI†). The adsorption capacity of 0.4 mg g−1 is for per unit of total weight including the CFS and TiO2 nanofibers. Because the mass fraction of TiO2 nanofibers in the products is about 13%, so the adsorption capacity for per unit weight of TiO2 nanofibers is calculated to nearly 3.1 mg g−1, which is 15 times that of P25 TiO2 (0.2 mg g−1) and higher than that (1.78 mg g−1) of macroscopic TiO2–BiOX frameworks.36 It is noted that the pure CFSs only causes a slight fading of the RhB solution (Fig. S5, ESI†). The high adsorption capacity of the TiO2 nanofiber/carbon fiber sheet benefited from its large BET surface area (17.638 m2 g−1) and pore structure (Fig. S6, ESI†). When the TiO2/carbon nanofiber sheet is irradiated with ultraviolet light (254 nm), conduction band electrons (e−) and valence band holes (h+) are generated on TiO2 nanofibers. The photogenerated electrons and holes can react with H2O and O2 molecules, leading to the formation of reactive oxygen species, such as O2− and ˙OH. The resulting reactive oxygen species can oxidize RhB to CO2, H2O and mineral end-products (Fig. S7, ESI†).37,38 After irradiation under UV light for 60 min, the diffuse reflection spectrum of the modified textile changed back to the original form (blue line), indicating that the adsorbed RhB molecules were removed. The adsorption–lifting–irradiation process can be repeated many times while retaining the photocatalytic activity of the modified textile (Fig. S8, ESI†), confirming that the modified textile is a reusable and durable photocatalytic material.Subsequently, we investigated the photodegradation behaviors of RhB under continuous photocatalysis of the modified textile. During the studies, the modified textile is immersed in the solution all the time. RhB was completely photodegraded by the modified textile within 80 min (Fig. 4a), demonstrating the high photocatalytic efficiency of the modified textile. RhB was degraded fast initially, but the degradation rate become slow after 40 min as the concentration of RhB decreased. The average degradation rate k is 0.6 mg h−1 (k = m/t, where m and t indicate the weight of the degraded pollutant and the irradiation time, respectively), which is similar with the reported TiO2 nanofiber framework.36 It is noted that the pure CFSs has no photocatalytic activity and it only causes a slight fading of the RhB solution due to the adsorption of a small amount of RhB by the textile (Fig. S7, ESI†).
Owing to the existence of carbon fibers, the as-prepared TiO2/carbon cloth exhibited lower photocatalytic activity than the reported TiO2 nanofiber membranes.20,23,26 For TiO2 nanofiber/carbon fiber sheets, only the TiO2 nanofibers can utilize ultraviolet light for photodegradation of RhB, because carbon fibers do not have photocatalytic activity. For TiO2 nanofiber membranes, all the building blocks of the membrane can serve as active sites to eliminate RhB. Furthermore, the TiO2 nanofibers/CFSs exhibited much higher photocatalytic activity than the reported TiO2 nanotubes/CFSs3 and P25/CFSs (Fig. S9a, ESI†). Comparing with TiO2 nanotubes and P25, TiO2 nanofibers possess ultralong 1D structure, which is benefiting for the overlapping and interweave with carbon fibers, so the TiO2 nanofibers were not only grown between the carbon fibers, but also formed on the surface of the carbon fibers, leading to higher loading capacity of TiO2 nanofibers on carbon fiber sheets (one square centimeter of CFS can support 25 mg nanofibers vs. 5 mg nanotubes or P25). In other word, more active sites were formed on TiO2 nanofibers/CFSs than TiO2 nanotubes/CFSs and P25/CFSs with same size. Consequently, The TiO2 nanofibers/CFSs exhibited much higher photocatalytic activity than the reported TiO2/CFSs composite. In addition, the modified textile had good reuse capacity; even after five times reuse, the degradation ratio of RhB is still as high as ca. 97% (Fig. 4c), while only 20% for P25/CFSs (Fig. S9b, ESI†). No catalyst loss was observed during the repeated photocatalytic test (Fig. 4d), indicating the TiO2 nanofibers were firmly anchored with the CFS. The TiO2/carbon cloth was also capable of photodecomposing many organic pollutes, including methylene blue, methyl orange, and fluorescein (Fig. S10, ESI†). Therefore, the modified textile is a class of recoverable and durable photocatalytic materials, which has great potential in water purification.
Conclusions
In the work reported here, we have demonstrated a facile strategy for the engineering growth of TiO2 nanofibers on CFS. The resulting large size nanofiber macroscopic structure is a durable photocatalytic material since it can be easily recovered and the robustness of the modified textile allows it to be reused. The as-prepared TiO2/carbon cloth possesses both adsorption functionality and cleaning functionality and should have potential for advanced water purification. In addition, the obtained nanofiber macroscopic structure is a kind of versatile scaffold for the construction of macroscopic multifunctional materials, which might also be of interest in solar cell, catalysis, and separation technology.Acknowledgements
This work was financially supported by the Natural Science Foundation of Zhejiang Province, China (LQ14B010002), and the Innovative Research Program for Postgraduates in Universities of Jiangsu Province for financial support.References
- R. I. McDonald, P. Green, D. Balk, B. M. Fekete, C. Revenga, M. Todd and M. Montgomery, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6312–6317 CrossRef CAS PubMed.
- Z. R. Wang, H. Wang, B. Liu, W. Z. Qiu, J. Zhang, S. H. Ran, H. T. Huang, J. Xu, H. W. Han and D. Chen, ACS Nano, 2011, 5, 8412–8419 CrossRef CAS PubMed.
- P. Chen, L. Gu, X. D. Xue, M. J. Li and X. B. Cao, Chem. Commun., 2010, 46, 5906–5908 RSC.
- B. Liu, J. Zhang, X. F. Wang, G. Chen, D. Chen, C. W. Zhou and G. Z. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef CAS PubMed.
- J. Chen, K. X. Sheng, P. H. Luo, C. Li and G. Q. Shi, Adv. Mater., 2012, 24, 4569–4573 CrossRef CAS PubMed.
- S. Zhang, Z. F. Lu, L. Gu, L. L. Cai and X. B. Cao, Nanotechnology, 2013, 24, 465202–465212 CrossRef PubMed.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101–5105 CrossRef CAS PubMed.
- S. Karan, Q. F. Wang, S. Samitsu, Y. Fujii and I. Ichinose, J. Membr. Sci., 2013, 448, 270–291 CrossRef CAS PubMed.
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770–4799 CrossRef CAS PubMed.
- Z. C. Wu, Z. H. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner and A. F. Hebard, Science, 2004, 305, 1273–1276 CrossRef CAS PubMed.
- X. B. Cao, D. P. Qi, S. Y. Yin, J. Bu, F. J. Li, C. F. Goh, S. Zhang and X. D. Chen, Adv. Mater., 2013, 25, 2957–2962 CrossRef CAS PubMed.
- Z. Ren, Z. Huang, J. Xu, J. Wang, P. Bush, M. Siegal and P. Provencio, Science, 1998, 282, 1105–1107 CrossRef CAS.
- C. V. Nguyen, L. Delzeit, A. M. Cassell, J. Li, J. Han and M. Meyyappan, Nano Lett., 2002, 2, 1079–1081 CrossRef CAS.
- H. B. Li, X. C. Gui, L. H. Zhang, S. S. Wang, C. Y. Ji, J. Q. Wei, K. L. Wang, H. W. Zhu, D. H. Wu and A. Y. Cao, Chem. Commun., 2010, 46, 7966–7968 RSC.
- X. C. Gui, A. Y. Cao, J. Q. Wei, H. B. Li, Y. Jia, Z. Li, L. L. Fan, K. L. Wang, H. W. Zhu and D. H. Wu, ACS Nano, 2010, 4, 2320–2326 CrossRef CAS PubMed.
- M. B. Bryning, D. E. Milkie, M. F. Islam, L. A. Hough, J. M. Kikkawa and A. G. Yodh, Adv. Mater., 2007, 19, 661–664 CrossRef CAS.
- H. Hu, Z. B. Zhao, W. B. Wan, Y. Gogotsi and J. S. Qiu, Adv. Mater., 2013, 25, 2219–2223 CrossRef CAS PubMed.
- S. Nardecchia, D. Carriazo, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, Chem. Soc. Rev., 2013, 42, 794–830 RSC.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- L. W. Zhu, L. Gu, Y. Zhou, S. L. Cao and X. B. Cao, J. Mater. Chem., 2011, 21, 12503–12510 RSC.
- S. P. Albu, A. Ghicov, J. M. Macak, R. Hahn and P. Schmuki, Nano Lett., 2007, 7, 1286–1289 CrossRef CAS PubMed.
- L. W. Zhu, H. X. Li, Z. G. Ren, H. F. Wang, W. Yao and J. P. Lang, RSC Adv., 2013, 3, 15421–15426 RSC.
- X. W. Zhang, T. Zhang, J. W. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731–3736 CrossRef CAS.
- X. B. Cao, Y. Zhou, J. Wu, Y. X. Tang, L. W. Zhu and L. Gu, Nanoscale, 2013, 5, 3486–3495 RSC.
- M. N. Chong, B. Jin, C. W. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- L. W. Zhu, Z. G. Ren and J. P. Lang, Chin. J. Chem., 2012, 30, 1469–1473 CrossRef CAS.
- L. W. Zhu, L. K. Zhou, H. X. Li, H. F. Wang and J. P. Lang, Mater. Lett., 2013, 95, 13–16 CrossRef CAS PubMed.
- Y. Ku, C.-M. Ma and Y.-S. Shen, Appl. Catal., B, 2001, 34, 181–190 CrossRef CAS.
- A. Bozzi, T. Yuranova and J. Kiwi, J. Photochem. Photobiol., A, 2005, 172, 27–34 CrossRef CAS PubMed.
- A. Bozzi, T. Yuranova, I. Guasaquillo, D. Laub and J. Kiwi, J. Photochem. Photobiol., A, 2005, 174, 156–164 CrossRef CAS PubMed.
- L. P. Yang, Z. Y. Liu, J. W. Shi, H. Hu and W. F. Shangguan, Catal. Today, 2007, 126, 359–368 CrossRef CAS PubMed.
- X. F. Wang, F. S. Han, X. Wei and X. F. Wang, Mater. Lett., 2010, 64, 1985–1988 CrossRef CAS PubMed.
- S. Meng, R. Jun and K. Efthimios, Nano Lett., 2008, 8, 3266–3272 CrossRef CAS PubMed.
- B. Tan and Y. Y. Wu, J. Phys. Chem. B, 2006, 110, 15932–15938 CrossRef CAS PubMed.
- K. Zhu, N. R. Neale, A. Miedaner and A. J. Frank, Nano Lett., 2007, 7, 69–74 CrossRef CAS PubMed.
- X. B. Cao, Z. F. Lu, L. W. Zhu, L. Yang, L. Gu, L. L. Cai and J. Chen, Nanoscale, 2014, 6, 1434–1444 RSC.
- M. Stylidi, D. I. Kondarides and X. E. Verykios, Appl. Catal., B, 2003, 40, 271–286 CrossRef CAS.
- T. S. Natarajan, M. Thomas, K. Natarajan, H. C. Bajaj and R. J. Tayade, Chem. Eng. J., 2011, 169, 126–134 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46601a |
| This journal is © The Royal Society of Chemistry 2014 |