The photodimerization characteristics of anthracene pendants within amphiphilic polymer micelles in aqueous solution†
Yan Liu,
Huan Chang,
Jinqiang Jiang*,
Xiangyang Yan,
Zhaotie Liu and
Zhongwen Liu
Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an, Shaanxi 710062, China. E-mail: jiangjq@snnu.edu.cn; Fax: +86-29-81530784; Tel: +86-29-81530784
First published on 27th May 2014
Abstract
An amphiphilic polymer with anthracene pendants of PEO-b-PAM has been applied to comprehensively investigate the photodimerization characteristics of anthracene in aqueous solution upon various selected narrow band of light irradiations in the UV-vis-NIR region.
Anthracene and its derivatives are interesting compounds because of their versatile photochemical applications.1 Owing to their diverse photophysical and photochemical properties, anthracenes have been used in many systems such as functional polymers, triplet sensitizers and molecular fluorosensors.1–5 Another well-known facet of anthracenes is their typical photodimerization reaction, which occurs favourably in UV light irradiations (250–400 nm) and has been widely investigated for the application of photoresponsive materials.6–15 Furthermore, the dimerization has been recently accomplished with visible light (400–700 nm) or multiphoton light in order to weaken some side effects during irradiation.16–18 It is widely accepted that the photodimerization efficiency always depends on the photochemical absorbency of anthracenes to a certain light irradiation. Thus, the photodimerization efficiency can be anticipated according to the characteristic UV-vis absorbance of anthracenes, and it can be easily controlled by adjusting the photon flux.6 However, to date, reports that comprehensively describe the photodimerization characteristics of anthracene upon various light irradiations in the UV-vis-NIR region are rare. Such reports, apparently, will help further understanding on the photodimerization mechanism of anthracenes, as well as other photo-crosslinkable chromophores, and may improve their opportunities in potential applications, such as nanofabrication, nanoreactors and biological functional models, especially when their photophysical and photochemical properties are demanded to be controlled sophisticatedly.19–22
Herein, based on our ongoing study of photoresponsive amphiphilic polymers, our attention was drawn to the photodimerization characteristics of anthracene pendants within the polymer micelles of poly(ethylene oxide)-b-poly(anthracene methyl methacrylate) (PEO-b-PAM), which was concisely synthesized by ATRP (Scheme 1). Scheme 1 illustrates the amphiphilic structure of such block copolymer and its photodimerization upon light irradiation in aqueous solution. Note that the photodimerization efficiency of anthracene always depends on its concentration and molecule mobility,23 and the typical amphiphilic block copolymer of PEO-b-PAM can spontaneously form aggregated micelles. Thus, anthracene pendants would be entangled into the confined micelle core with high local concentration and restricted mobility, which may facilitate the dimerization at the start of irradiation and result in the maximum equilibrium extent in the late stages of irradiation.
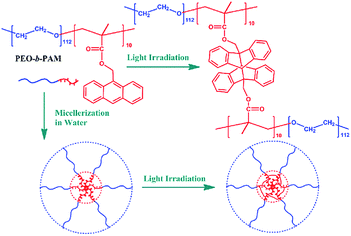 | ||
| Scheme 1 Schematic illustration of the amphiphilic polymer with anthracene pendants (PEO-b-PAM) and its photodimerization upon light irradiation in aqueous solution. | ||
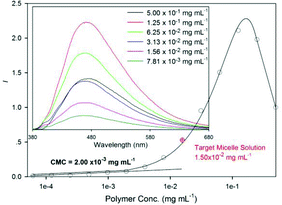 | ||
| Fig. 1 Normalized fluorescence emission intensity of anthracene at 470 nm (λexc = 353 nm). Inset: fluorescence emission spectra of PEO-b-PAM in aqueous solution. | ||
We first focused on the micellization of PEO-b-PAM in aqueous solution. The obtained polymer micelle aqueous solution was first diluted gradually to evaluate its stability, and the dilution process was traced by fluorescent spectroscopy and DLS instrument. As shown in the inset of Fig. 1, the anthracene emission spectra in aqueous solution are easily detected with broad fluorescence emission covering 380–680 nm intervals. The maximum emission peak around 470 nm is not always weaken during the dilution. In fact, it is strengthened at the beginning and then becomes weak upon further dilution. This phenomenon can often be found in the assemblies of anthracene-containing polymers.13 The self-quenching effect of anthracene decreases with the decreasing concentration of anthracene pendants first, and then the fluorescence decreases with the further decrease in the concentration of anthracene.24,25 Furthermore, as shown in Fig. 1, the plot of normalized maximum emission below polymer concentration of 2.00 × 10−3 mg mL−1 shows a relatively gentle and linear decrease along with the dilution, while the DLS examinations (S2 in ESI†) show narrow size distributions around 50 nm above 2.00 × 10−3 mg mL−1 and large fluctuations below this concentration. Thus, we assigned this value as the critical micelle concentration (CMC) of PEO-b-PAM in aqueous solution. The nanostructures of polymer micelles before and after photodimerization were also visualized by AFM images with uniformly spherical micelles (S3 in ESI†). Taking both the micelle stability and the demands of the real-time UV-vis spectroscopy into consideration, the polymer concentration used in photodimerization was kept at 0.015 mg mL−1.
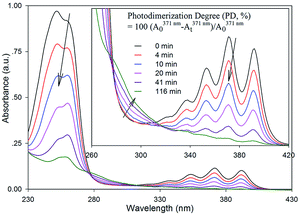
In order to comprehensively investigate the photodimerization characteristics of anthracene pendants in polymer micelles, an adjustable double grating monochromator of Omni-λ1805i equipped with a broadband laser-driven light source of EQ-1500 LDLS™ and a set of six optical filters was assembled to afford the adjustable narrow band wavelength of light between 250 and 950 nm. Under the used conditions, 1 mL of polymer micelle solution (0.015 mg mL−1) was sealed in the fluorescence cuvette and photodimerization of anthracene pendants was performed directly on an optical bench at 25 °C with gentle stirring. As designed, the anthracene micelle solution was first photodimerized with 371 ± 5 nm (1.20 mW cm−2) wavelength light, which corresponded to one of the characteristic absorbance bands of anthracene pendants in aqueous solution. As shown in Fig. 2, the UV-vis spectrum before irradiation showed a broad absorption band around 260 nm, and a series of vibrationally spaced absorption structures at 320, 338, 353, 371 and 392 nm (finger like absorption bands), which are typical of anthracenes.9 During irradiation, accompanied with the weak ascending between 280 and 310 nm, all the absorbance bands decreased significantly, strongly indicating the ongoing photodimerization of anthracene in the polymer micelle core.9 Moreover, because of its linear degradation and higher signal-to-noise ratio, the absorption band intensity at 371 nm was used as the baseline to measure the time-dependent photodimerization changes, resulting in the definition of photodimerization degree (PD) by comparing the peak absorption at 371 nm.
As shown in Fig. 3a, upon 371 ± 5 nm light irradiation, the time-dependant PD values for anthracene pendants synchronously decreased with the lowering of irradiation intensities. For 1.20 mW cm−2 irradiation, it showed saturating behaviour, affording a linear quick increase in the initial stage (up to 40%), a relatively slow increase thereafter (from 40% to 70%), and finally levelled off above 90%. The time-dependences of these observed PD changes upon various irradiation intensities are fit reasonably with a biexponential equation:
| PD(t) = X1(1 − e−k1t) + X2(1 − e−k2t) | (1) |
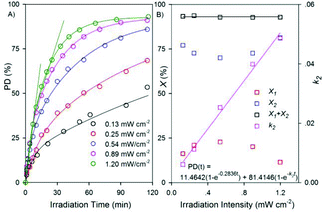 | ||
| Fig. 3 (a) The time-dependent PD of anthracenes upon 371 ± 5 nm light irradiation with different intensities. The solid curves are four parameter biexponential fits to eqn (1). (b) The intensity dependence of X1, X2, X1 + X2 values according to the four parameters biexponential fitting, and the linear dependence of k2 according to the one parameter biexponential fitting. | ||
In order to better understand the photodimerization kinetics dependency of irradiation conditions, and according to the characteristic absorbance bands of anthracene pendants in aqueous solution, another five narrow band UV light of 261, 300, 338, 353, 392 ± 5 nm and four vis-NIR light of 416, 550, 700 and 900 ± 5 nm were chosen as the irradiation light. As shown in Fig. 4a, when irradiated by these various wavelengths from the double grating monochromator with the same slits (their irradiation intensities corresponding to the highest values in Fig. 4b), the obtained time dependences of PD are fit very well to the four parameter biexponential of eqn (1), and all the UV light irradiations gave faster dimerization rates and higher dimerization extents than the vis-NIR light at the same time. The solid linear lines in Fig. 4b are zero-order linear fits of X1 + X2 values obtained by changing the irradiation intensities of the above selected light. All the X1 + X2 values obtained by the UV light irradiations were above 90%, despite there being large gaps in light absorbency of the selected UV light according to the characteristic absorbance bands of anthracenes (see the curve line in Fig. 4b). Even for the latter four vis-NIR light irradiations, in which region anthracenes have nearly no absorptions, they still afforded considerable efficiencies with PDmax values of 55%, 35%, 25% and 32% for 416, 550, 700 and 900 ± 5 nm light irradiations, respectively, which can be ascribed to the multi-photon effect. These results strongly show that the photodimerization extent (PDmax) of anthracenes always favours UV light rather than vis-NIR light and is independent of the intensities of a certain light irradiation.
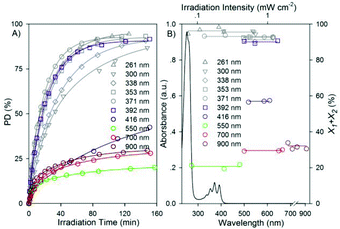 | ||
| Fig. 4 (a) The time-dependence of PD upon selected narrow band light irradiations derived from the monochromator with the same slits. The solid curves are four parameter biexponential fits to eqn (1). (b) The UV-vis-NIR absorbance spectrum of polymer micelles (0.015 mg mL−1) and the zero-order intensity dependence of X1 + X2 values for the selected light irradiations. | ||
To summarize, we have synthesized an amphiphilic block copolymer of PEO-b-PAM with anthracene pendants, which can afford the high local concentration and restricted mobility of anthracene pendants in the confined micelle core and thus facilitate the dimerization of anthracenes upon light irradiations. Most efforts have been dedicated to the photodimerization of anthracenes; however, to the best of our knowledge, our study is the first attempt to comprehensively describe it using various narrow band wavelength light in the UV-vis-NIR region. The result shows that the photodimerization of anthracene pendants occurs more favourably in UV light than vis-NIR light, and for a certain light irradiation, the dimerization extent that anthracenes can reach is always independent of light intensities. This indicates that anthracenes cannot afford the similar photodimerization efficiencies of UV light by simply strengthening the power of vis-NIR light.
Acknowledgements
This work was supported by the Nature Science Foundation of China (NSFC 21374056), the Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-10-0452), the Fundamental Research Funds for the Central Universities (GK201302045), the Program for Changjiang Scholars Innovative Research Team in University (IRT 1070), the Shaanxi Innovative Research Team for Key Science and Technology (2012KCT-21, 2013KCT-17) and the One Hundred Plan of Shaanxi Province.Notes and references
- H.-D. Becker, Chem. Rev., 1993, 93, 145–172 CrossRef CAS.
- H. Bouas-Laurent, A. Castellan, J.-P. Desvergne and R. Lapouyade, Chem. Soc. Rev., 2001, 30, 248–263 RSC.
- C. Schäfer, R. Eckel, R. Ros, J. Mattay and D. Anselmetti, J. Am. Chem. Soc., 2007, 129, 1488–1489 CrossRef PubMed.
- B. Fabre, D.-M. Bassani, C.-K. Liang, S. Lhenry and P. Hapiot, J. Phys. Chem. C, 2013, 117, 12725 CAS.
- J.-H. Huang, J.-H. Su and H. Tian, Mater. Chem., 2012, 22, 10977–10989 RSC.
- H. Kihara and M. Yoshida, ACS Appl. Mater. Interfaces, 2013, 5, 2650–2657 CAS.
- C. Wang, D.-Q. Zhang, J.-F. Xiang and D.-B. Zhu, Langmuir, 2007, 23, 9195–9200 CrossRef CAS PubMed.
- R.-O. Al-Kaysi and C.-J. Bardeen, Adv. Mater., 2007, 19, 1276–1280 CrossRef CAS.
- M. Ikegami, I. Ohshiro and T. Arai, Chem. Commun., 2003, 1566–1567 RSC.
- X.-L. Ye, X.-S. Jiang, B. Yu, J. Yin and P. Vana, Biomacromolecules, 2012, 13, 535–541 CrossRef CAS PubMed.
- S.-R. Jezowski, L.-Y. Zhu, Y.-B. Wang, A.-P. Rice, G.-W. Scott, C.-J. Bardeen and E.-L. Chronister, J. Am. Chem. Soc., 2012, 134, 7459–7466 CrossRef CAS PubMed.
- W. Chen, J.-Y. Wang, W. Zhao, L. Li, X.-Y. Wei, A.-C. Balazs, K. Matyjaszewski and T.-P. Russell, J. Am. Chem. Soc., 2011, 133, 17217–17224 CrossRef CAS PubMed.
- Z.-L. Su, B. Yu, X.-S. Jiang and J. Yin, Macromolecules, 2013, 46, 3699–3707 CrossRef CAS.
- J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Org. Lett., 2013, 15, 6148–6151 CrossRef CAS PubMed.
- Y.-J. Zheng, M. Micic, S.-V. Mello, M. Mabrouki, F.-M. Andreopoulos, V. Konka, S.-M. Pham and R.-M. Leblanc, Macromolecules, 2002, 35, 5228–5234 CrossRef CAS.
- J.-T. Good, J.-J. Burdett and C.-J. Bardeen, Small, 2009, 5, 2902–2909 CrossRef CAS PubMed.
- R.-R. Islangulov and F.-N. Castellano, Angew. Chem., Int. Ed., 2006, 45, 5957–5959 CrossRef CAS PubMed.
- A.-S. Dvornikov, H. Bouas-Laurent, J.-P. Desvergneb and P.-M. Rentzepis, J. Mater. Chem., 1999, 9, 1081–1084 RSC.
- H. Wang, J. Zhuang and S. Thayumanavan, ACS Macro Lett., 2013, 2, 948–951 CrossRef CAS.
- M. Palacios, O. García and R.-H. Juan, Langmuir, 2013, 29, 2756–2763 CrossRef CAS PubMed.
- X.-R. Wang, G.-H. Liu, J.-M. Hu, G.-Y. Zhang and S.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 3138–3142 CrossRef CAS PubMed.
- M. Krishnamurthy, A. Dugan, A. Nwokoye, Y.-H. Fung, J.-K. Lancia, C.-Y. Majmudar and A.-K. Mapp, ACS Chem. Biol., 2011, 6, 1321–1326 CrossRef CAS PubMed.
- S. Arumugam, D.-R. Vutukuri, S. Thayumanavan and V. Ramamurthy, J. Photochem. Photobiol., A, 2007, 185, 168–171 CrossRef CAS PubMed.
- B. Yu, X.-S. Jiang and J. Yin, Soft Matter, 2011, 7, 6853–6862 RSC.
- H. Mori, I. Tando and H. Tanaka, Macromolecules, 2010, 43, 7011–7020 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Polymer synthesis, FL spectra, DLS, AFM investment of polymer micelles, and the photodimerization procedures. See DOI: 10.1039/c4ra02315c |
| This journal is © The Royal Society of Chemistry 2014 |