One pot synthesis of graphene quantum disks derived from single-layered exfoliated graphene sheets and their application in bioimaging†
I-Wen Peter Chen*a,
Yi-Wen Chenb,
Yang-Hsiang Chanc,
Sheng-Hong Saint Jhoua and
Yu-Wei Zhanga
aDepartment of Applied Science, National Taitung University, Taitung City 95002, Taiwan. E-mail: iwchen@nttu.edu.tw; Fax: +886 89 342539
bDepartment of Industrial Engineering and Enterprise Information, Tunghai University, Taichung City 40704, Taiwan
cDepartment of Chemistry, National Sun Yat-sen University, 70 Lien Hai Road, Kaohsiung, Taiwan 80424
First published on 29th May 2014
Abstract
A novel procedure involving pyridinium-assisted hydrothermal synthesis has been developed for the preparation of monodisperse graphene quantum disks (GQDs) with a homogeneous size. Due to the high water solubility and pH stability, the first application of GQDs in the bioimaging of HeLa cells has been demonstrated.
Graphene is a novel two-dimensional single-layered nanomaterial that has opened new horizons in fundamental science and advanced technology.1–3 The interest in this material arises because of its unique electronic, chemical, and electromechanical properties and its potential applications in areas such as electrode materials, catalysis, and sensing, among others.4–7 Graphene quantum disks (GQDs) are graphene materials with a size of less than 100 nm, and they combine a quantum confinement effect with photoluminescence (PL) properties.8–11 Such GQDs display cell permeability, and non-toxicity, which can potentially be used in vitro and in vivo applications in the fields of cellular imaging, biological labelling, and drug delivery.10,12–14 As a result of these unique properties, several methods have been established to prepare GQDs, including hydrothermal, acid treatment, and electrochemical synthesis methods.12 It should be mentioned that GQDs synthesized by chemical cutting methods mostly exhibit polydispersion not only in diameter but also number of layers because it is so difficult to precisely define the starting and end sites and pathways of cutting raw materials of GQDs such as carbon fibers, and carbon nanotubes.15 Besides, layered stacking and lateral aggregation also give rise to a broad distribution in both diameter and number of layered GQDs throughout the chemical cutting processes. Moreover, there is a lack of useful separation methods to separate fully or predominantly monolayer GQDs from natural polydispersive samples because the as-synthesized GQDs with any layered number are highly hydrophilicity that the technique of high-speed centrifugation become ineffective. With this aim in mind, the raw material would be an important factor that influences the layered structure and lateral size of GQDs.12,16 A novel procedure involving pyridinium-assisted hydrothermal synthesis has been developed for the preparation of nearly monodisperse GQDs with a homogeneous size. To the best of our knowledge, the preparation of monodisperse GQDs using single-layered exfoliated graphene sheets (EGSs) have not been explored to date.
Herein, we report a facile route for the cutting of single-layered EGSs into GQDs. The synthesis route for GQDs is shown in Fig. 1. Our approach consists of three steps. Step 1, the raw material for this process was HOPG, which were obtained by mechanical cutting of 1 mg HOPG (1 cm × 1 cm). The HOPG were mixed with 1 M Py+Br3− in water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Step 2, the mixture was sonicated in a water bath for 45 min. The EGSs were freely dispersed and suspended in aqueous solution.17 Step 3, the EGSs suspension was involved hydrothermal reaction at 200 °C and 500 psi for 3 h to synthesize GQDs. Our new methodology allows preparing nearly monodisperse and predominantly monolayer GQDs with high stability at room temperature.
1). Step 2, the mixture was sonicated in a water bath for 45 min. The EGSs were freely dispersed and suspended in aqueous solution.17 Step 3, the EGSs suspension was involved hydrothermal reaction at 200 °C and 500 psi for 3 h to synthesize GQDs. Our new methodology allows preparing nearly monodisperse and predominantly monolayer GQDs with high stability at room temperature.
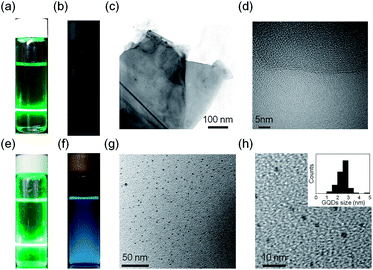
The PL properties of EGSs and GQDs were studied using both a green-light laser pointer. Owing to the Tyndall effect, a track can clearly be observed in Fig. 2a and e as the laser beam passed through the EGSs suspension and GQDs solution, respectively. Moreover, the suspension of the EGSs and the GQDs can be stable for more than a year without significant precipitation. The EGSs were characterized by transmission electron microscopy (TEM), as shown in Fig. 2c. The TEM image of EGSs shows exfoliation of single-layered sheets owing to the Py+-assisted exfoliation process, and the measured sheet diameter of EGSs was approximately 500 nm. The high-resolution TEM (HR-TEM) image reveals that single-layered EGSs were successfully produced as shown in Fig. 2d. When the PL measurement was performed on a sample in water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the EGSs suspension did not show any PL emission (Fig. 2b) under irradiation with 365 nm UV lamp (4 W). The raw material of GQDs is a single-layered EGSs rather than any other carbon source such as graphene oxide,18 reduced graphene oxide, graphite nanofibers,19 and graphite nanoparticles.8 The as-prepared EGSs were further treated by a hydrothermal method to prepare single-layered GQDs. A strong blue emission was observed on excitation of the solution of GQDs by irradiation with 365 nm UV lamp (Fig. 2f). A TEM image of GQDs is shown in Fig. 2g. More TEM images are provided in the ESI (Fig. S1†). HR-TEM shows that GQDs are circular in shape and have uniform particle size distribution in the range of 2 nm and 4 nm (2.5 ± 0.6 nm average diameter) (Fig. 2h). AFM characterization was performed to demonstrate the single-layered nature in GQDs synthesized from the EGSs suspension. The AFM results show that the thickness of majority of the synthesized GQDs was approximately 0.8 nm (Fig. S2†), corresponding to single-layered GQDs.20
1), the EGSs suspension did not show any PL emission (Fig. 2b) under irradiation with 365 nm UV lamp (4 W). The raw material of GQDs is a single-layered EGSs rather than any other carbon source such as graphene oxide,18 reduced graphene oxide, graphite nanofibers,19 and graphite nanoparticles.8 The as-prepared EGSs were further treated by a hydrothermal method to prepare single-layered GQDs. A strong blue emission was observed on excitation of the solution of GQDs by irradiation with 365 nm UV lamp (Fig. 2f). A TEM image of GQDs is shown in Fig. 2g. More TEM images are provided in the ESI (Fig. S1†). HR-TEM shows that GQDs are circular in shape and have uniform particle size distribution in the range of 2 nm and 4 nm (2.5 ± 0.6 nm average diameter) (Fig. 2h). AFM characterization was performed to demonstrate the single-layered nature in GQDs synthesized from the EGSs suspension. The AFM results show that the thickness of majority of the synthesized GQDs was approximately 0.8 nm (Fig. S2†), corresponding to single-layered GQDs.20
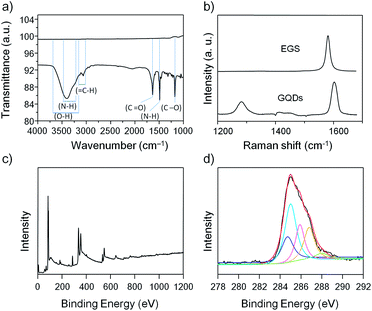
Several instrumental techniques were used to confirm successful preparation of the GQDs. The EGSs and GQDs samples were studied by FTIR, and the GQDs showed a broad and intense hydroxyl peak at 3402 cm−1, an aromatic C–H stretching band at 3062 cm−1, an amide peak at 1635 cm−1, a peak at 1489 cm−1 arise result from the N–H bending vibration, and ester C–O stretch at 1175 cm−1, whereas no peaks were observed for the EGSs sample (Fig. 3a). Raman spectroscopy is one of the most direct and nondestructive techniques for characterization of sp2 and sp3 carbon atoms in graphene. The Raman spectrum of graphene at approximately 1300 cm−1 shows the so-called disorder-induced mode (D band). The intensity of the D band indicates the abundance of defects in the graphene. The characteristic C–C stretching band in graphene represents the Raman-allowed tangential mode (G band), which is an intrinsic characteristic of sp2 carbons. EGSs shows an intense G band peak centered at approximately 1580 cm−1 and a D band peak, which one would expect at approximately 1300 cm−1, is not observed. The intensity ratio ID/IG is a reliable indicator to verify changes from sp2 carbons to defect forms of sp3 configuration in graphene. After the hydrothermal reaction, oxygen-containing and nitrogen-containing groups were introduced into the edges of GQDs. Thus, the as-prepared GQDs gave rise to an observable D band in the Raman spectrum. The ID/IG ratios of EGSs and GQDs were 0.004 and 0.45, respectively (Fig. 3b). The ID/IG ratios of GQDs indicate that the as-prepared GQDs are highly crystalline and are the best quality than reported results.15 X-ray photoelectron spectroscopy (XPS) was used to probe surface compositions of GQDs. The XPS survey spectrum of GQDs shows a graphitic C 1s peak at 285.0 eV, an O 1s peak at approximately 532 eV and N 1s peak at approximately 400 eV for GQDs (Fig. 3c), indicating that GQDs are composed of C, N, and O. Also, the residue of the pyridinium moiety and Br− counterion can be also observed in the XPS survey spectrum. The other peaks in the spectrum are because of the binding energy of Au as an internal standard. The XPS survey spectrum of GQDs contains an N 1s peak and shows an N/C atomic ratio of 17.9%. The C1s spectrum for GQDs (Fig. 3d) can be deconvoluted into several peaks that correspond to C–C (284.5 eV), C–N (286.0 eV), C–O (286.5 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.0 eV) functional groups, which are consistent with the FTIR results. It can be seen from Fig. S3† that the main peak at 402 eV is attributed to the formation of N 1s of an amide bonding, indicating that the decomposed pyridinium is attached to aromatic rings of GQDs. The minor peak at 399.8 eV is ascribed to the N–C bond of free pyridinium.17 Therefore, it can be concluded that the as-prepared GQDs are composed of sp2 aromatic and C–N species derived by oxygen-containing and pyridinium derivatives along with abundant amide, carbonyl/carboxylate and hydroxyl groups on the GQDs.
O (288.0 eV) functional groups, which are consistent with the FTIR results. It can be seen from Fig. S3† that the main peak at 402 eV is attributed to the formation of N 1s of an amide bonding, indicating that the decomposed pyridinium is attached to aromatic rings of GQDs. The minor peak at 399.8 eV is ascribed to the N–C bond of free pyridinium.17 Therefore, it can be concluded that the as-prepared GQDs are composed of sp2 aromatic and C–N species derived by oxygen-containing and pyridinium derivatives along with abundant amide, carbonyl/carboxylate and hydroxyl groups on the GQDs.
The optical properties of EGSs and GQDs were measured to show the effect of surface chemistry on the PL behaviour. The UV-vis spectra of GQDs are shown in Fig. 4a and there is no observable peak, but it come up with a long absorption band. Irradiation of the sample with a 365 nm handheld UV lamp led to a strong blue luminescence with a peak at around 411 nm (Fig. 2f). To investigate the excitation-dependent PL properties of GQDs, a detailed PL study was performed with various excitation wavelengths. The PL peaks were redshifted from 411 to 531 nm (Fig. 4b). The GQDs displayed an excitation wavelength that was dependent on PL properties and this behaviour was particularly marked when the excitation wavelength was above 350 nm. Some suggested that emissive traps, free zigzag sites, and electronic conjugate structures are the reasons for the phenomenon.13 However, the photoluminescence mechanism of GQDs still remain to be identified. The GQDs showed excellent photostability on exposure to light from a 150 W xenon lamp with 365 nm UV light. After irradiation with UV light for 4 h, the measured PL intensity of GQDs only decreased by 8% and the PL intensity became stable after 1 h (inset of Fig. 4b).
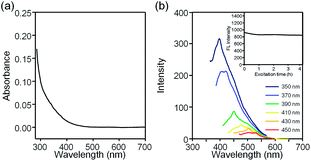 | ||
| Fig. 4 The optical properties and PL stability of the aqueous solution of GQDs. (a) UV-vis absorption spectra. (b) The excitation-dependent PL behaviour. Inset shows the photostability of the GQDs. | ||
To gain a better understanding of the dependence of the PL on the chemical environment of GQDs, the changes in PL intensity on varying the pH value were studied in the pH range 2 to 12. The PL intensities of GQDs over the pH range studied are shown in Fig. S4.† The pH-dependent PL results show that GQDs emit stable PL at pH > 7, whereas under acidic conditions the PL intensity gradually decreases to 25%. This phenomenon can be understood by considering the situation at different pH values. The structures of GQDs in acidic solution are protonated which decreases the electrostatic repulsions between each single-layered GQDs, thus limiting the dispersion through layer–layer stacking.16,19 Consequently, the emissive state of GQDs is quenched in PL. The GQDs functional groups are highly stable on irradiation with UV light over a wide range of ionic strengths,21 indicating that the as-synthesized GQDs are suitable candidates for biological applications.
As described above, the as-synthesized GQDs are nanoscale material that has great potential for biological applications such as cell imaging. To demonstrate the capability of GQDs for cell imaging, an in vitro experiment was performed to image HeLa cells by laser scanning confocal microscope. After cell incubation with GQDs for 4 h, the resulting bright field image clearly shows the HeLa cells (Fig. 5a) and the confocal fluorescence image shows GQDs distributed around each nucleus (Fig. 5b). These results show that GQDs can label both the membrane and cytoplasm of the HeLa cells without entering the nucleus to a significant extent. It can therefore be stated that GQDs were taken up by the HeLa cells and distributed within the cytoplasm. These results prove that the GQDs derived from single-layered EGS have great potential as a probe for cell imaging.
 | ||
| Fig. 5 Fluorescence images of living cancer cells, HeLa, after incubation with GQDs for 4 h. (a) HeLa cells under bright field and (b) HeLa cells on excitation at 408 nm. | ||
In conclusion, a facile route to synthesize water-soluble GQDs has been developed. The resulting GQDs also have a uniform size of approximately 2.5 nm. It was demonstrated that the synthesized GQDs can directly penetrate into HeLa cells without influencing their differentiation capacity and they show PL properties. These properties were exploited in the successful imaging of living HeLa cells. Furthermore, the high water-solubility, tunable PL of the GQDs means that they have great potential for applications in bioimaging.
Acknowledgements
The authors gratefully acknowledge funding from the NSC, Taiwan (101-2113-M-143-003-MY2), and National Taitung University for financial and research support. Thanks to Ms. C.-Y. Chien, S.-J. Ji and C.-C. Chen of Precious Instrument Center (NTU) for the assistance in SEM, TEM and XPS experiments.Notes and references
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192 CrossRef CAS PubMed.
- H. Y. Mao, S. Laurent, W. Chen, O. Akhavan, M. Imani, A. A. Ashkarran and M. Mahmoudi, Chem. Rev., 2013, 113, 3407 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156 CrossRef CAS PubMed.
- J. K. Wassei and R. B. Kaner, Acc. Chem. Res., 2013, 46, 2244 CrossRef CAS PubMed.
- D. K. James and J. M. Tour, Acc. Chem. Res., 2013, 46, 2307 CrossRef CAS PubMed.
- C. Su and K. P. Loh, Acc. Chem. Res., 2013, 46, 2275 CrossRef CAS PubMed.
- F. Liu, M.-H. Jang, H. D. Ha, J.-H. Kim, Y.-H. Cho and T. S. Seo, Adv. Mater., 2013, 25, 3657 CrossRef CAS PubMed.
- G. Eda, Y.-Y. Lin, C. Mattevi, H. Yamaguchi, H.-A. Chen, I.-S. Chen, C.-W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2011, 133, 15221 CrossRef CAS PubMed.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953 CrossRef CAS PubMed.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J.-J. Zhu, Nanoscale, 2013, 5, 4015 RSC.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436 CrossRef CAS PubMed.
- Q. Xue, H. Huang, L. Wang, Z. Chen, M. Wu, Z. Li and D. Pan, Nanoscale, 2013, 5, 12098 RSC.
- R. Ye, C. Xiang, J. Lin, Z. Peng, K. Huang, Z. Yan, N. P. Cook, E. L. G. Samuel, C.-C. Hwang, G. Ruan, G. Ceriotti, A.-R. O. Raji, A. A. Martí and J. M. Tour, Nat. Commun., 2013, 4, 2943 Search PubMed.
- I-W. P. Chen, C.-Y. Huang, S.-H. S. Jhou and Y.-W. Zhang, Sci. Rep., 2014, 4, 3928 Search PubMed.
- S. Zhu, J. Zhang, S. Tang, C. Qiao, L. Wang, H. Wang, X. Liu, B. Li, Y. Li, W. Yu, X. Wang, H. Sun and B. Yang, Adv. Funct. Mater., 2012, 22, 4732 CrossRef CAS.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J.-J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844 CrossRef CAS PubMed.
- D. Graf, F. Molitor, K. Ensslin, C. Stampfer, A. Jungen, C. Hierold and L. Wirtz, Nano Lett., 2007, 7, 238 CrossRef CAS PubMed.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03488k |
| This journal is © The Royal Society of Chemistry 2014 |