Synthesis of polymeric lubricating films directly at the sliding interface via mechanochemical reactions of allyl alcohols adsorbed from the vapor phase
Abstract
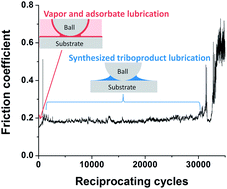
Ensuring lubrication effects during disruption of lubricant molecule supply is an important objective for boundary lubrication. Friction tests conducted in an allyl alcohol vapor environment yielded a friction coefficient near 0.25, which was significantly lower than the values (>0.6) measured for unlubricated contacts, and produced a tribopolymer where contact occurred. No tribopolymer was created outside the contact area, and the underlying substrate was not worn inside the contact area. A tribopolymer film created during 800 cycles of allyl alcohol vapor flow could lubricate sliding contacts for 30 000 cycles even after allyl alcohol vapor supplied was ceased. No significant wear or friction increase was observed during these cycles. Infrared spectroscopy analysis of the slide contact area indicated that the tribopolymer is a polyalcohol formed by polymerization of the allylic group. No tribopolymer formation was observed for vapor phase lubrication with saturated normal alcohols. The tribopolymer created from allyl alcohol vapor must be mechanochemically synthesized within the sliding interface. Such a mechanochemical synthesis of polymeric lubricant film could be applied to boundary lubrication of micro-scale devices.
- This article is part of the themed collection: Tribology

 Please wait while we load your content...
Please wait while we load your content...