A highly efficient and recyclable molybdate sulfuric acid (MSA) catalyst for the synthesis of dimethyl (2,3-dihydro-1H-inden-6-ylamino) (substituted) methylphosphonates under microwave irradiation†
Abstract
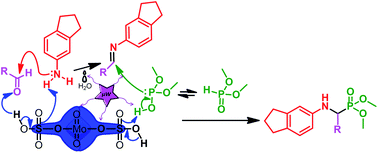
An efficient green synthesis of dimethyl (2,3-dihydro-1H-inden-6-ylamino) (substituted) methylphosphonates has been achieved under solvent-free conditions by the reaction of 2,3-dihydro-1H-inden-5-amine, aldehydes and ethyl dimethyl phosphonate by microwave irradiation in the presence of molybdate sulfuric acid (MSA) as a catalyst. High product yields in shorter reaction times, easy isolation of products, reusability of solid catalysts and environmentally benign reaction conditions are its advantages.

 Please wait while we load your content...
Please wait while we load your content...