High pressure CO2-controlled reactors: enzymatic chiral resolution in emulsions
Abstract
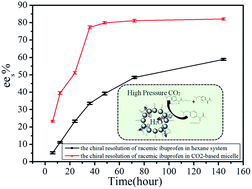
In this work we have reported the formulation of a CO2-based micelle stabilized by nontoxic TMN series surfactants. Enantioselection of racemic ibuprofen catalyzed by Candida antarctica lipase B (CALB) was used as a model reaction. The effect of reactive parameters, such as temperature, pH, pressure, and water content on reactive environment and conversion has been discussed. For the resolution of racemic ibuprofen in CO2-based micelles, the enzymatic activity reached a high level at 45 °C, with pressure 250 bar, pH 7.4, and water to surfactant ratio W0 25. In addition, the relatively long-chain length in TMN-10 could help the esterification and trans-esterification processes, which resulted in an efficient reaction rate in a CO2-based micelle system. Enzymatic catalysis has been conducted in a CO2-based system rather than in the conventional media to make the enzyme reaction greener. The better resolution efficiency in high pressure CO2-based micelles could be achieved within a relatively short period of time compared with other traditional reactive systems.

 Please wait while we load your content...
Please wait while we load your content...