Binder-free cathodes based on sulfur–carbon nanofibers composites for lithium–sulfur batteries†
Songtao Lua,
Yan Chena,
Xiaohong Wu*a,
Zhida Wanga,
Lingyuan Lva,
Wei Qin*b and
Lixiang Jiangc
aDepartment of Chemistry, Harbin Institute of Technology, Harbin, 150001, PR China. E-mail: wuxiaohong@hit.edu.cn; Fax: +86 451 86402522; Tel: +86 451 86402522
bSchool of Materials Science and Engineering, Harbin Institute of Technology, Harbin, 150001, PR China. E-mail: qinwei@hit.edu.cn; Fax: +86 451 86402522; Tel: +86 451 86402522
cScience and Technology on Reliability and Environmental Engineering Laboratory, Beijing Institute of Satellite Environment Engineering, Beijing, 100094, PR China. E-mail: lyjiang001@163.com; Fax: +86 10 68745576; Tel: +86 10 68746751
First published on 7th April 2014
Abstract
Binder-free S-FCNF composite films with sulfur coated uniformly on the surface of FCNFs were used as cathodes for lithium–sulfur batteries. Such cathodes have both high sulfur content (78 wt%) and large areal mass loading (8 mg cm−2), and they can deliver a high areal specific capacity of 1.87 mA h cm−2 (234 mA h gelectrode−1) after 100 cycles at 0.1 C.
Rechargeable lithium sulfur batteries are based on the redox couple of S8 + 16Li = 8Li2S and have output voltage ∼2.2 V.1–4 According to this couple, sulfur has a high theoretical gravimetric capacity of ∼1675 mA h g−1 and a high theoretical energy density of ∼2600 W h kg−1, which are both substantially higher than commercial cathode materials (for example, ∼150 mA h g−1 for layered oxides and ∼170 mA h g−1 for LiFePO4).5–8 Combined with other advantages of sulfur (low toxicity and naturally abundant), lithium–sulfur battery is a promising technology for the next generation energy storage and has attractive applications, especially for electric and hybrid vehicles.
Despite these numerous advantages, there are still several barriers for the communization of lithium sulfur batteries. The first one is the low electrical conductivity of sulfur (5 × 10−30 S cm−1), the second one is the huge volume expansion during discharge due to the differences in volume densities of Li2S (1.67 g cm−3) and S (2.03 g cm−3), the third one is the dissolution and diffusion of lithium polysulfide intermediates that are formed during the charge–discharge process. These problems will cause loss of active sulfur, short cycle life, limited specific capacity, and low coulombic efficiency of Li–S batteries.9–19
In order to address these barriers, many efforts have been made. According to current papers, a widely pursued approach to solve these problems is to trap polysulfides by using physical barriers and/or polymer absorbers.1,2,12,15,19–27 As a result, high specific capacities of more than 800 mA h g−1 have been reported by many groups.12,18,19,26–28 However, the commercialized application of the Li–S system hasn't been realized yet, largely because most of the as-prepared electrodes have low areal sulfur loading (typically less than 1 mg cm−2). In addition, to the best of our knowledge, the highest percentage of sulfur in the cathode is still less than 75 wt%.11,12,15,18,19,27,29 As a result, the areal specific capacities of electrodes fabricated by previous works were mostly under 1.0 mA h cm−2, which greatly decrease the usable capacity of a battery system.11,15,27,30
During our recent work on coaxial graphene wrapping of sulfur coated functionalized carbon nanofibers (S-FCNFs) for high performance lithium sulfur batteries,21 we found that S-FCNFs composites themselves could form binder-free films and showed excellent performance as the cathodes even at both high sulfur content and large areal mass loading (78 wt%, 8 mg cm−2). As a result, lithium sulfur batteries assembled using such cathodes had improved areal specific capacities. They were able to deliver a reversible areal specific capacity of 4.49 mA h cm−2 (561 mA h gelectrode−1) at 0.1 C and maintain 1.87 mA h cm−2 (234 mA h gelectrode−1) even after 100 charge–discharge cycles.
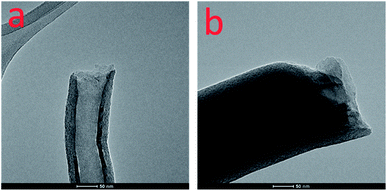
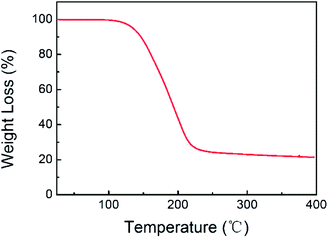
Fig. 1 shows typical transmission electron microscopy (TEM) images for FCNFs without (a) and with (b) sulfur coating. As can be seen from these two images, a continuous and uniform tubular layer of sulfur was coated on the surface of FCNFs and formed nanomicrosphere structure. The increases in diameter of FCNFs after sulfur coating indicate that the sulfur layer had thicknesses around 45.5 nm. On the basis of thermogravimetric analysis (TGA, Fig. 2), about 78 wt% S was loaded into S-FCNFs.
The cathode for Li–S batteries testing was prepared by pressing a piece of a freestanding film (with typical areal mass loading of 8.0 mg cm−2) onto a piece of nickel mesh. Unlike the conventional sulfur electrode preparation approach that involves substantial amount of carbon additive and binder, the approach used in this work is binder-free and could reduce the total weight of an electrode. The test batteries were assembled as coin cells (type 2032), with lithium foil used as the negative electrodes. The charge–discharge tests were measured with 0.1 C (where 1 C corresponds to a current density of 1675 mA g−1) and voltage window of 1.5–3.0 V. Typical voltage profiles were shown in Fig. 3 and are in good agreement with those reported previously. As can be seen, two plateaus at 2.35 V and 2.05 V were clearly observed during the discharge process, which correspond to the formation of long-chain lithium polysulfides (Li2Sx, 4 ≤ x ≤ 8) and short-chain lithium polysulfides (such as Li2S2 and Li2S), respectively. On the basis of the discharge results, a high areal specific discharge capacity of 4.49 mA h cm−2 (561 mA h gelectrode−1) was obtained during the initial discharging process.
The electrode exhibited well-overlapped and flat plateaus with continued cycling (Fig. 4a), suggesting good stability and reversibility. Fig. 4b compares the capacity of the electrode as a function of cycle numbers. At a rate of 0.1 C, an initial areal specific discharge capacity of 4.49 mA h cm−2 (561 mA h gelectrode−1) was observed. The discharge capacities had large dropping in the following few cycles due to loss of active sulfur. However, the capacity was quickly stabilized and exhibited a reversible areal specific capacity of 1.87 mA h cm−2 (234 mA h gelectrode−1) after 100 cycles, which could be attributed to the absorption of polysulfides intermediates by the FCNFs. Furthermore, at the same time, as can be seen from the Fig. 4c, the coulombic efficiency remained at around 98% for all the S-FCNFs cathodes during cycling, showing another promising property of the S-FCNFs cathodes.
Conclusions
In summary, we have successfully synthesized a novel binder-free cathode by effectively coating FCNFs with sulfur. In this S-FCNFs structure, the highly conductive FCNFs act as electrical conductor and accommodating the volume expansion of sulfur during discharge and can absorb polysulfides intermediates in the cathode, which significantly improved cyclic stability of Li–S batteries. As a result, a reversible and satisfied specific capacity of 1.87 mA h cm−2 (234 mA h gelectrode−1) was obtained after 100 cycles at 0.1 C, showing promising performance for lithium–sulfur batteries.Financial support from the Nature Science Foundation of China (no. 51078101, 51173033), and the Fundamental Research Funds for the Central Universities (no. HIT.BRETIII.201224 and 201312).
Notes and references
- X. L. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500 CrossRef CAS PubMed.
- P. G. Bruce, L. J. Hardwick and K. M. Abraham, MRS Bull., 2011, 36, 506 CrossRef CAS.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19 CrossRef CAS PubMed.
- X. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821 RSC.
- G. Y. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462 CrossRef CAS PubMed.
- C. D. Liang, N. J. Dudney and J. Y. Howe, Chem. Mater., 2009, 21, 4724 CrossRef CAS.
- D. Peramunage and S. Licht, Science, 1993, 261, 1029 CAS.
- X. L. Li, Y. L. Cao, W. Qi, L. V. Saraf, J. Xiao and Z. M. Nie, J. Mater. Chem., 2011, 21, 16603 RSC.
- Y. L. Cao, X. L. Li, I. A. Aksay, J. Lemmon, Z. M. Nie and Z. G. Yang, Phys. Chem. Chem. Phys., 2011, 13, 7660 RSC.
- R. Elazari, G. Salitra, A. Garsuch, A. Panchenko and D. Aurbach, Adv. Mater., 2011, 23, 5641 CrossRef CAS PubMed.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288 CrossRef CAS PubMed.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904 CrossRef CAS PubMed.
- L. W. Ji, M. M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. G. Zhang, Energy Environ. Sci., 2011, 4, 5053 CAS.
- L. W. Ji, M. M. Rao, H. M. Zheng, L. Zhang, Y. C. Li and W. H. Duan, J. Am. Chem. Soc., 2011, 133, 18522 CrossRef CAS PubMed.
- N. W. Li, M. B. Zheng, H. L. Lu, Z. B. Hu, C. F. Shen and X. F. Chang, Chem. Commun., 2012, 48, 4106 RSC.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee and T. Bein, Angew. Chem., Int. Ed., 2012, 51, 3591 CrossRef CAS PubMed.
- H. L. Wang, Y. Yang, Y. Y. Liang, J. T. Robinson, Y. G. Li and A. Jackson, Nano Lett., 2011, 11, 2644 CrossRef CAS PubMed.
- F. Wu, J. Z. Chen, L. Li, T. Zhao and R. J. Chen, J. Phys. Chem. C, 2011, 115, 24411 CAS.
- Y. Yang, G. H. Yu, J. J. Cha, H. Wu, M. Vosgueritchian and Y. Yao, ACS Nano, 2011, 5, 9187 CrossRef CAS PubMed.
- L. W. Ji, M. M. Rao, H. M. Zheng, L. Zhang, Y. C. Li and W. H. Duan, J. Am. Chem. Soc., 2011, 133, 18522 CrossRef CAS PubMed.
- S. T. Lu, Y. Cheng, X. Wu and J. Liu, Nano Lett., 2013, 13, 2485 CrossRef CAS PubMed.
- G. M. Zhou, L. C. Yin, D. W. Wang, L. Li, S. F. Pei and I. R. Gentle, ACS Nano, 2013, 7, 5367 CrossRef CAS PubMed.
- G. L. Xu, Y. F. Xu, J. C. Fang, X. X. Peng, F. Fu and L. Huang, ACS Appl. Mater. Interfaces, 2013, 5, 10782 CAS.
- T. Xu, J. X. Song, M. L. Gordin, H. S. Sohn, Z. X. Yu and S. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 11355 CAS.
- L. Wang, D. Wang, F. X. Zhang and J. Jin, Nano Lett., 2013, 13, 4206 CrossRef CAS PubMed.
- G. M. Zhou, D. W. Wang, F. Li, P. X. Hou, L. C. Yin and C. Liu, Energy Environ. Sci., 2012, 5, 8901 CAS.
- Y. S. Su and A. Manthiram, Chem. Commun., 2012, 48, 8817 RSC.
- J. C. Guo, Z. C. Yang, Y. C. Yu, H. D. Abruna and L. A. Archer, J. Am. Chem. Soc., 2013, 135, 763 CrossRef CAS PubMed.
- S. S. Zhang and J. A. Read, J. Power Sources, 2011, 10, 76 CrossRef PubMed.
- Y. S. Su and A. Manthiram, Nat. Commun., 2012, 3, 1166 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the material preparation, characterization, and device testing. See DOI: 10.1039/c4ra02122c |
| This journal is © The Royal Society of Chemistry 2014 |