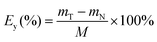Liquid exfoliation of Zn–Al layered double hydroxide using NaOH/urea aqueous solution at low temperature
Yong Wei,
Fucheng Li and
Lan Liu*
College of Materials Science and Engineering, Key Lab of Guangdong Province for High Property and Functional Macromolecular Materials, South China University of Technology, Guangzhou 510641, PR China. E-mail: psliulan@scut.edu.cn; Fax: +86 20 87114857; Tel: +86 20 87114857
First published on 27th March 2014
Abstract
The present study provides a new simple but efficient method for preparation of layered double hydroxide (LDH) nanosheets. The exfoliation of Zn–Al LDH is demonstrated in NaOH/urea aqueous solution at low temperature below zero Celsius (−10 °C) to form stable transparent colloidal suspensions. The successful exfoliation of LDH is proved by XRD, SEM, TEM and AFM measurements. Typically, the thickness of exfoliated LDH nanosheets is around 0.6 nm while the pristine Zn–Al LDH is about 300 nm. The further study of the mechanism indicates that the NaOH hydrates formed at low temperature below zero Celsius could easily intercalate into the interlayer galleries of LDH and attach to the host layers and the urea hydrates formed at low temperature below zero Celsius play a key role in aggregation prevention and nanosheets stabilization. These two hydrates result in breakage of the original hydrogen bonding network between the adjacent LDH layers and the buildup of a new hydrogen bonding network between the hydrates and LDH host layers. The approach adopted to exfoliate LDH in this paper is a completely novel method, which is extremely convenient, highly efficient and eco-friendly.
1. Introduction
Materials with two-dimensional structures, such as layered double hydroxide nanosheets, have been widely studied in recent decades owing to their intriguing physicochemical properties.1–3 Layered double hydroxides, usually abbreviated as LDHs, also known as hydrotalcite-like clays, are a class of the lamellar clay family consisting of host layers and guest species in the interlayer galleries. The LDH materials can be described as [MII1−xMIIIx(OH)2]x+(An−)x/n·mH2O. In this general formula, MII and MIII represent bivalent and trivalent metal cations respectively, in which the atom radius of these cations is essentially equal; x is the mole ratio of MIII/(MII + MIII), which is always range from 0.17 to 0.33;4 An− is the intercalated charge-balancing anions.5–7More recently, much attention has been drawn to prepare highly dispersed layered LDH nanosheets and its extended materials due to LDH nanosheets have extensive applications in the field of catalytic materials,8–12 reinforced nanocomposites,13 barrier materials,14 dyes and pigments,15 biomaterials,16 electrode materials,17,18 and photoelectric materials,19 etc. However, between the two adjacent host layers exist very compact hydrogen bond, van der Waals force and electrovalent bond,20–24 making it full of challenge to obtain highly dispersed LDH nanosheets. In the past ten years, tremendous efforts have been made to exfoliate LDH to nanosheets. For example, Hibino and coworkers have reported that LDH intercalated with amino acid (glycine, serine, or L-asparagine, etc.) could be exfoliated in formamide (HCONH2) at room temperature.25 The study demonstrated formamide is a general solvent to exfoliate various LDHs such as [Mg–Al], [Zn–Al], [Co–Al] and [Ni–Al].26,27 The author thought that LDH layers modified by amino acid, especially glycine, became very attractive to formamide, which will form new hydrogen bond with LDH host layers, and the penetration of adequate formamide resulted in exfoliation of LDH. Up to now, the combination of homogeneous precipitation and formamide is considered as the most optimum method to prepare LDH nanosheets.28 Nonetheless, some MII–MIII LDHs cannot be synthesized by homogeneous precipitation method. To solve this problem, Ma et al. developed topochemical synthesis of these LDHs and exfoliated them in formamide successfully.5,29–33 In spite of well-defined LDH nanosheets could be obtained using formamide as solvent, this method is somewhat time-consuming (sometimes even one month) and not eco-friendly enough. Water, as an absolutely environmentally friendly solvent, was reported to exfoliate LDH containing short chain carboxylates such as lactate and acetate anions.34 It is commonly believed that LDH involving these anions displays swelling behavior in water and form transparent colloidal suspension, which is the reason for the delamination of LDH in water. However, it is reported that the key to successful delamination using hydrothermal method is to use the freshly prepared LDH; the LDH after vacuum drying cannot be exfoliated at all.35 The short chain alcohol (ethanol, butanol, amyl alcohol and hexanol) was also reported to delaminate LDH with the help of dodecyl sulfate (DS), sodium octyl benzene sulfonate (SOBS) or sodium dodecyl benzene sulfonate (SDBS).36,37 But the mechanism of exfoliation in alcohol is absolutely different from that in water and formamide, the authors suggested that if the interlayer water molecules could be replaced by short chain alcohol, the exfoliatation occurred while the opposite not. However, the long time mechanical and ultrasonic treatment, refluxing, and low yield make this method undesired. Besides, acrylates, DMSO, CCl4, toluene, N,N-dimethylformamide–ethanol mixtures were used as solvent to exfoliate various LDHs as well.38–42 Parts of these approaches could obtain well defined LDH nanosheets under ambient temperature or normal heating process yet numerous deficiencies were also existed at the same time.
Alkali/urea aqueous solution system is a very interesting solvent system, which was reported to dissolve cellulose that has strong inter- and intra-molecular hydrogen bond and intact crystalline structure.43,44 It is well known that at low temperatures, “NaOH hydrates” could be easily attracted to cellulose chains to form a new hydrogen bonding network, and then the “urea hydrates” may be self-assembled at the surface of the NaOH hydrogen-bonded cellulose to form an inclusion complex, which lead to the dissolution of cellulose.45–47 That means the NaOH/urea aqueous solution could destroy the hydrogen bond and crystalline structure of cellulose at low temperature in a very short time (about just 2 min).48,49 To the best knowledge of us, the structure of LDH and cellulose has much in common. For example, there are a large number of hydroxyl groups on the host layers of LDH and molecular chain of cellulose, thus strong hydrogen bond are formed both in LDH and cellulose. Theoretically, the hydrogen bond and lamellar structure of LDH could also be destroyed using NaOH/urea aqueous solution at low temperatures.
Herein, the Zn–Al LDH was exfoliated in 6–9 wt% NaOH/10–15 wt% urea mixed aqueous solution at low temperature below zero (−10 °C) and stable transparent colloidal suspensions of exfoliated LDH were obtained. The exfoliation of Zn–Al LDH is proved by XRD, SEM, TEM, and AFM measurements. The further investigation of the exfoliation mechanism indicates that the formed NaOH hydrates in subzero environment could easily intercalate into interlayer regions of LDH and attach on the host layers, contributing to breakage of original hydrogen bonding network and formation of new hydrogen bonding network. Then urea hydrates self-assembled at the surface of NaOH hydrogen-bonded host layers of LDH, stabilizing the colloidal suspension. Therefore, LDH nanosheets could be obtained at low temperature using alkali/urea aqueous solution in a very short time. As far as we know, the exfoliation method of Zn–Al LDH used in this study is a completely novel approach which is not reported in the previous papers. This study successfully present formation of Zn–Al LDH nanosheets and provided a new simple method for preparation of Zn–Al LDH nanoplates which is extremely convenient (without any heat, ultrasound or refluxing), rapid (only 3 min), high-efficiency (about 5 wt%) and environmentally friendly.
2. Experimental section
2.1. Materials
ZnCl2·6H2O and AlCl3·6H2O were purchased from TianJin Damao chemical reagents factory (TianJin, China). Solid urea and NaOH were bought from TianJin Fuchen chemical reagents factory (TianJin, China). All the chemicals were analytical grade and used without any other purification. Deionized water was prepared ourselves, particularly.2.2. Synthesis of brucite-like Zn–Al LDH
Urea or hexamethylenetetramine (HMT) hydrolysis method,33,50 chemical coprecipitation method,51,52 gas–liquid contacting method,53 templating synthesis method,18,54 and many other methods were adopted to synthesis various LDHs. In this study, the LDH containing Zn and Al was synthesized using the urea hydrolysis method due to its highly economy, convenience and regularity. In a typical procedure, 70 mmol ZnCl2·6H2O and 30 mmol AlCl3·6H2O was dissolved in 200 mL deionized water to form transparent solution, then 120 mmol solid urea was added to the transparent solution, after the dissolution of solid urea, the clear mixed solution was reflux heated at 105 °C under stirring at 270 rpm. One hour later, turn down the stir and keep the system at 95 °C for 24 hours. The Zn–Al LDH powder was obtained by centrifugation and washed with deionized water at least 3 times and then freeze drying.2.3. Exfoliation of Zn–Al LDH using NaOH/urea aqueous solution at low temperature
A series of NaOH/urea aqueous solution with different NaOH and solid urea concentration was prepared in beaker at room temperature and then precooled in refrigerator to −10 °C. Next, a certain quality of as prepared Zn–Al LDH were added to the mixed solution at once and stirred vigorously for 3 min. The colorless and transparent exfoliated Zn–Al LDH colloidal suspension was obtained by centrifuging the suspension under 2000 rpm for 10 min to remove the not fully exfoliated LDH, all the procedures were operated below ambient temperature. To make sure the low temperature is important to exfoliate LDHs, the identical experiments are also operated at 0 °C, 5 °C, 25 °C and 60 °C.2.4. Characterization
X-ray diffraction (XRD) data of pristine LDH, exfoliated LDH nanosheets and self-assembled LDH aggregates regenerated from anhydrous ethanol bath were collected by Bruker D8 ADVANCE diffractometer (Bruker Co. Ltd., Billerica, America) with Ni-filtered Cu-Kα radiation (λ = 0.15418 nm) in the range of 2–70° with a scanning speed of 2° min−1 at 40 kV and 40 mA. The XRD samples of exfoliated LDH nanosheets were obtained by centrifuging the transparent colloidal suspensions at a speed of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min, some glue-like aggregate was recovered from the suspension. The XRD samples of regenerated LDH aggregates from anhydrous ethanol bath were prepared as follows: the colorless and transparent Zn–Al LDH nanosheets suspension was added to anhydrous ethanol dropwise and then a large number of floccules-like precipitate was observed immediately, then the white slurry was centrifuged under 5000 rpm for 10 min and freeze drying.
000 rpm for 30 min, some glue-like aggregate was recovered from the suspension. The XRD samples of regenerated LDH aggregates from anhydrous ethanol bath were prepared as follows: the colorless and transparent Zn–Al LDH nanosheets suspension was added to anhydrous ethanol dropwise and then a large number of floccules-like precipitate was observed immediately, then the white slurry was centrifuged under 5000 rpm for 10 min and freeze drying.
Scanning electron microscopy (SEM) analysis was carried out using a Nano SEM 430 instrument (FEI Co. Ltd., Hillsboro, America). To avoid charging effect, all the LDH samples were sprayed with a thin platinum layer before SEM observation.
Transmission electron microscopy (TEM) images were obtained by JEOL JEM-2100HR instrument (EDAX Inc., Mahwah, America), accelerating voltage was operated at 200 kV. For TEM characterization, the regenerated exfoliated LDH nanosheets were dispersed in anhydrous ethanol and homogenized in an ultrasonic bath. A drop of final supernate was deposited on copper grids with continuous carbon films and air dried at ambient temperature.
Atom force microscopy (AFM) measurements were performed by using NanoScope (R) III instrument (Bruker Co. Ltd., Billerica, America) operated in a tapping mode. The samples for AFM images were prepared by dispersing the LDH sample in anhydrous ethanol with ultrasonic bath and dropped onto freshly mica surface and allowed them to dry at room temperature.
Fourier Transform Infrared Spectroscopy (FTIR) spectra was recorded on a Bruker VERTEX70 FTIR spectrometer (Bruker Co. Ltd., Billerica, America) at ambient temperature using KBr disc technique between 4000 and 400 cm−1.
3. Results and discussion
3.1. Structure characterization of LDH samples
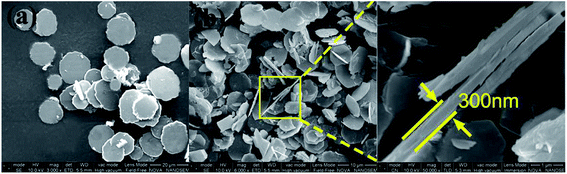
In this research, the highly regularity and crystallinity disk-like platelets of Zn–Al LDH were synthesized by urea slow hydrolysis process. As the SEM images shown (Fig. 1), the average diameter of the typical disk-like pristine Zn–Al LDH is 6–12 μm and the thickness is about 300 nm. The XRD curve of pristine LDH clearly demonstrates the highly crystallinity of the LDH sample (Fig. 2). The interlamellar spacing d is calculated through Bragg equation from: λ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, which is 0.78 nm. This regular structure makes it contribution to the following exfoliation procedure.
θ, which is 0.78 nm. This regular structure makes it contribution to the following exfoliation procedure.
 | ||
| Fig. 2 XRD patterns of pristine Zn–Al LDH sample and insert is enlarged view of XRD pattern in high angles. | ||
3.2. Exfoliation of Zn–Al LDH at low temperature
In the exfoliation procedure, the highly colorless and transparent exfoliated LDH colloidal suspensions was obtained by adding a certain of pristine Zn–Al LDH into low temperature below zero Celsius (−10 °C) NaOH/urea aqueous solution and stirring vigorously for 3 min, and then centrifuging the LDH suspension under 2000 rpm for 10 min to remove the not fully exfoliated LDH. To investigate the relationship of exfoliation efficiency (Ey) to NaOH concentration and urea concentration, a series of NaOH/urea aqueous solutions were tested in this research. In this study, Ey is defined aswhere mT is the total mass added in the NaOH/urea aqueous solution, mN is the mass of not fully exfoliated LDH sample obtained by centrifuging the suspension under 2000 rpm for 10 min and the precipitate was air dried at room temperature, M is the mass of NaOH/urea aqueous solution.
Fig. 3 shows the three dimensional phase diagram for exfoliation efficiency (Ey) of Zn–Al LDH in NaOH/urea aqueous solution at −10 °C. The surface plotting reveals that NaOH plays a critical role in exfoliation process. The Zn–Al LDH dispersed in precooled (−10 °C) 12 wt% urea aqueous solution was muddy and turbid while the same concentration of LDH in precooled (−10 °C) 8 wt% NaOH aqueous solution was transparent. In other words, precooled NaOH aqueous solution may be used to delaminate LDH relatively ideally while precooled urea aqueous solution almost could not (Fig. 4(a)α and β). However, using precooled NaOH aqueous solution to delaminate LDH is not recommended as the obtained Zn–Al LDH nanosheets suspensions without attendance of urea is extremely unstable in our experiment, in which precipitate appeared in short time. Stable and transparent LDH nanosheets suspensions could be obtained only combination of NaOH and urea. The results suggest that urea is not involved the delamination process directly but plays an important role in aggregation prevention and stabilizing the LDH nanosheets suspension (Fig. 4(a)γ). A large number of experimental results demonstrate the stable LDH nanosheets suspension can be successfully prepared when NaOH concentration from 6–9 wt% and urea from 10–15 wt%.
 | ||
| Fig. 3 Three dimensional phase diagram for exfoliation efficiency of Zn–Al LDH in precooled (−10 °C) NaOH/urea aqueous solution (Ey represents exfoliation efficiency). | ||
Besides, in Zhang and coworkers' research,45 cellulose can be dissolved in 4.2 wt% LiOH/12 wt% urea and 7 wt% NaOH/12 wt% urea aqueous solution but cannot be dissolved in 9.8 wt% KOH/12 wt% urea aqueous solution. The other two alkalis were also tried to exfoliate Zn–Al LDH in our experiment, what is the difference is that all these three alkali hydroxide could be used to exfoliate LDH. From this point, we can come to the conclusion that the mechanism of exfoliation of LDH is different from the cellulose dissolved in alkali hydroxide/urea aqueous solution at low temperature.
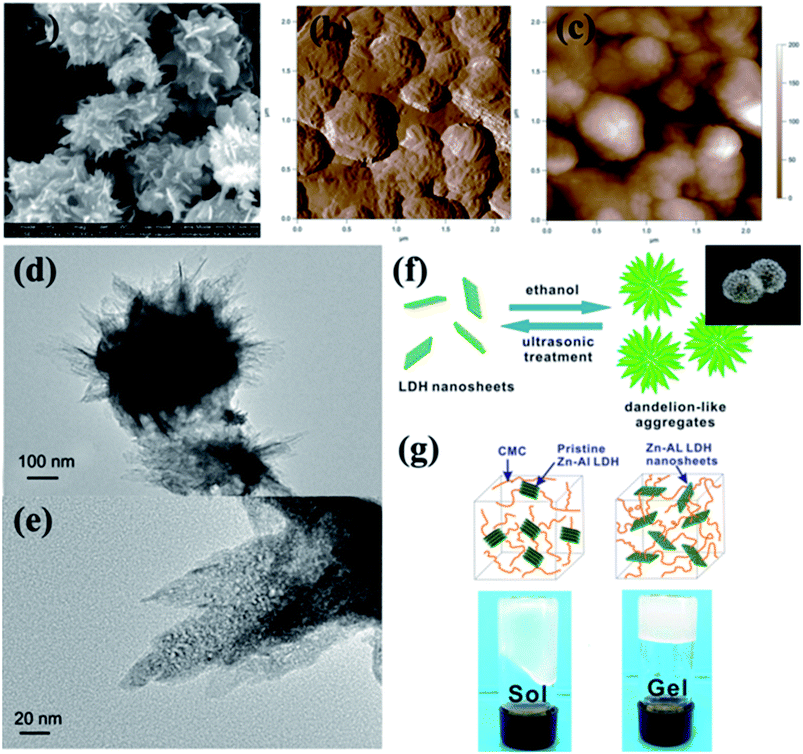
The XRD curve of exfoliated Zn–Al nanosheets powders obtained after centrifugation from LDH colloidal suspension was shown in Fig. 4(b). The sharp basal diffraction peaks were almost absent in this pattern, indicating the collapse of the ordered layered structure of the pristine Zn–Al LDH. The two-dimensional tapping mode AFM images (Fig. 5(a) and (b)), SEM image (Fig. 5(c)) and TEM image (Fig. 5(d)) of the exfoliated Zn–Al LDH nanosheets reveal the average lateral size of the nanosheets is about dozens of nanometers. Insert of Fig. 5(b) displays the height profile along the white line in image (Fig. 5(b)), which reveals the average height of the nanosheets is around 0.6 nm while the pristine LDH is about 300 nm (Fig. 1), indicating single layered LDH nanosheets can be obtained. All this characterization results demonstrate that the Zn–Al LDH was successfully exfoliated into nanosheets in NaOH/urea aqueous solution at low temperature.
To make sure the low temperature is important to exfoliate LDHs, the influence of the temperature on the exfoliation of LDH was studied. The degree of exfoliation in the colloidal suspensions can be indirectly estimated from the relative peak intensity of the regenerated dandelion-like aggregates from XRD patterns. The colloidal suspensions produced by exfoliation of LDH at different temperatures (−10 °C, 0 °C, 5 °C, 25 °C and 60 °C) were dropped into anhydrous ethanol bath to generate dandelion-like aggregates. Fig. 6 is the XRD patterns of dandelion-like aggregates. It is shown that, as the temperature drops, the intensity of the peak at 11.4° and 23.3° corresponding to (003) and (006) are greatly weakened. The presence of the two peaks was attributed to the regular arrangement of the adjacent two layers in the LDH. The peak intensity measures the regularity of the LDH layers. If the LDH were exfoliated into single layers existed randomly, the peaks nearly disappeared. In the regenerated dandelion-like aggregates at −10 °C, the peak intensity is obviously weaker than the other four peaks, demonstrating the lower regularity in the regenerated LDH structure. It is due to that the exfoliation degree is larger under the low temperature condition.
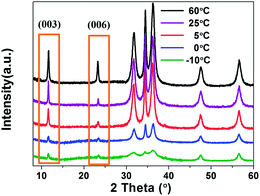 | ||
| Fig. 6 XRD patterns of regenerated Zn–Al LDH dandelion-like aggregates by dropping colloidal suspensions produced by different temperatures into anhydrous ethanol bath. | ||
Fig. 7(a–e) show SEM, AFM and TEM images of regenerated dandelion-like Zn–Al LDH aggregates from transparent colloidal suspensions (exfoliated at low temperature). The diameter of dandelion-like aggregates is around 500 nm. The surface of dandelion-like aggregates is not smooth as the dandelion-like aggregates are self-assembled by Zn–Al LDH nanoplates. The self-assembly aggregates can partly disaggregate to nanosheets dispersions under ultrasonic treatment (Fig. 7(e)). This is a very important method to produce LDH nanosheets aggregates, which has significant applications in various fields. For example, the re-obtained Zn–Al LDH nanosheets may provide physically crosslinking points for anionic polymer such as sodium carboxymethyl cellulose (CMC) via ionic bond and hydrogen bond (Fig. 7(g)), which has much potential applications in smart hydrogel. In addition, the LDH nanosheets may be a robust candidate for catalytic materials, electrode materials, photoelectric materials, etc.
To investigate any other precipitators, a few common organic solvents were tried in this study; we discovered methanol, dimethylsulfoxide (DMSO) and acetone which could regenerate Zn–Al LDH nanosheets as well. To the best knowledge of us, the reason about the regeneration is: in the present of these organic solvents, the hydrogen bond equilibrium between LDH nanosheets and formed NaOH and urea hydrates was broken. So the NaOH and urea hydrates attached on the LDH nanosheets were removed, the uncovered LDH nanosheets will aggregate to decrease their surface energy as much as possible.
3.3. The mechanism of exfoliation of LDH in NaOH/urea aqueous solution at low temperature
The rapid exfoliation of LDH in NaOH/urea aqueous solution is a very surprising phenomenon. It is well known that almost all the exfoliation processes are carried out under ambient temperature or normal heating pathway in previous works; hence it is very worthwhile to clarify the mechanism of the exfoliation.There are three interaction forces between the adjacent two LDH host layers: hydrogen bond, van der Waals force and electrovalent bond. We think the hydrogen bond is relatively more dominate in these three forces, for the quantity of hydrogen bond is proportionable to the positive ions (total of MII and MIII) while the van der Waals force and electrovalent bond are all limited by distance. So the success of exfoliation of LDH in NaOH/urea aqueous solution is attributed to the breakage of original hydrogen bond networks and formation of new hydrogen bond networks.
The change of the hydrogen bond network was detected by Fourier Transform Infrared Spectroscopy (FTIR), which is shown as Fig. 8. The strong and broad peaks at 3440 cm−1 belong to the stretching mode of hydroxyl group (–OH) on the LDH host layers. The presence of an obvious shoulder peak at around 3000 cm−1 in pristine LDH spectrum is attributed to the strong hydrogen bond network inside the interlayer galleries of the LDH. The disappearance of this shoulder peak after exfoliation suggests that the hydrogen bond network is destroyed, and the weak peaks associated with the bending mode of the water molecule at around 1640 cm−1 shift to the lower wavenumbers after exfoliation indicates the hydrogen bond is weaken, too.
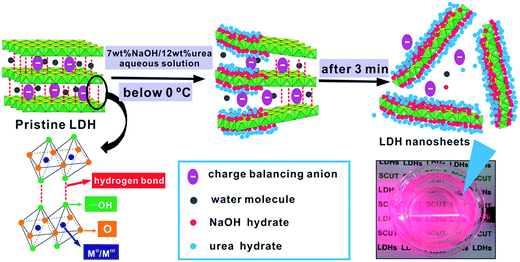
It is generally accepted that there will form “hydrates” in alkali and urea solution at low temperature below zero Celsius, which is crucial to explain the reason why LDH could be well exfoliated at low temperature in alkali/urea aqueous solution. Generally speaking, NaOH hydrates and urea hydrates will be formed respectively when NaOH/urea aqueous solution was cooled to 0 °C or even lower.46 The NaOH hydrates intercalate into the interlayer spaces of the LDH and attach on the host layers, therefore new hydrogen bond network was built between NaOH hydrates and hydroxyl group on the host layers of LDH. Urea hydrates could not directly attach on the LDH host layers, but they can easily self-assembled at the surface of NaOH hydrogen-bonded host layers of LDH, stabilizing the LDH nanosheets suspension and preventing the aggregation (Fig. 9). The combination of NaOH and urea makes the original hydrogen bond network of adjacent layers of LDH destroyed, which leads to the rapid exfoliation of LDH.
Nevertheless, it is worth noting that the dissolution of cellulose and exfoliation of LDH is different. Zhang and coworkers45 have reported that cellulose could easily dissolved in 4.2 wt% LiOH/12 wt% urea and 7 wt% NaOH/12 wt% urea aqueous solution but cannot be dissolved in 9.8 wt% KOH/12 wt% urea aqueous solution.45 They think the cations also take part in the dissolution process, and the Li+ and Na+ can form two hydration shells, while K+ can only form loose first hydration shell, therefore K+ cannot form stable complex with cellulose. But it seems that this argument is unsuitable for the exfoliation of LDH. In our experiment, the precooled 9.8 wt% KOH/12 wt% urea aqueous can also perfectly exfoliate Zn–Al LDH to nanosheets. This is properly because interspace of adjacent two layers of LDH is much larger than the adjacent two cellulose molecular chains, which makes K+ (with larger cationic radius than Li+ and Na+) cannot enter the inside of cellulose while can easily intercalate the interlamination of LDH.
4. Conclusions
In this paper, liquid exfoliation of Zn–Al LDH with highly crystallinity and regularity in alkali solution at low temperature below zero Celsius was carried out and Zn–Al LDH nanosheets were obtained. Our experimental results demonstrate that: (1) Zn–Al LDH could be easily exfoliated to LDH nanosheets by using 6–9 wt% NaOH and 10–15 wt% urea mixed aqueous solution at low temperature and the whole process just take a few minutes; (2) the exfoliation of LDH should be operated in subzero environment. (3) NaOH, LiOH and KOH could also be used to exfoliate LDH combined with urea at low temperature below zero Celsius; (4) the efficiency of liquid exfoliation can be higher than 5 wt%; (5) the exfoliated LDH could be “regenerated” from the colorless and transparent LDH nanosheets suspensions under the help of methanol, anhydrous ethanol, DMSO, acetone. With these solvents, the dandelion-like exfoliated LDH powder could be obtained but ethanol is identified as the optimal precipitator for its easily volatility and environmental benefits.Acknowledgements
The authors gratefully acknowledge National Nature Science Foundation of China (contact grant number: U1134005), Science and Technology innovation key project of universities of Guangdong province (CXZD1106), and Strategic new industry core technology research project of Guangdong province (2012A090100017) for the financial support.References
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef.
- A. K. Geima and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef.
- R. Z. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082–5104 CrossRef CAS.
- P. J. Sideris, U. G. Nielsen, Z. H. Gan and C. P. Grey, Science, 2008, 321, 113–117 CrossRef CAS.
- R. Z. Ma, Z. P. Liu, K. Takada, N. Iyi, Y. Bando and T. Sasaki, J. Am. Chem. Soc., 2007, 129, 5257–5263 CrossRef CAS.
- Z. P. Liu, R. Z. Ma, Y. Ebina, N. Iyi, K. Takada and T. Sasaki, Langmuir, 2007, 23, 861–867 CrossRef CAS.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS.
- B. F. Chen, F. B. Li, Z. J. Huang, T. Lu, Y. Yuan, J. L. Yu and G. Q. Yuan, RSC Adv., 2012, 2, 11449–11456 RSC.
- Y. F. Zhao, M. Wei, J. Lu, Z. L. Wang and X. Duan, ACS Nano, 2009, 3(12), 4009–4016 CrossRef CAS.
- L. He, Y. Q. Huang, A. Q. Wang, X. D. Wang, X. W. Chen, J. J. Delgado and T. Zhang, Angew. Chem., Int. Ed., 2012, 51, 6191–6194 CrossRef CAS.
- J. K. Zhao, Y. F. Xie, W. J. Yuan, D. X. Li, S. F. Liu, B. Zheng and W. G. Hou, J. Mater. Chem. B, 2013, 1, 1263–1269 RSC.
- K. Teramura, S. Iguchi, Y. Mizuno, T. Shishido and T. Tanaka, Angew. Chem., Int. Ed., 2012, 51, 8008–8011 CrossRef CAS.
- M. Kotal and S. K. Srivastava, J. Mater. Chem., 2011, 21, 18540–18551 RSC.
- D. Y. Wang, A. Das, A. Leuteritz, R. N. Mahaling, D. Jehnichen, U. Wagenknecht and G. Heinrich, RSC Adv., 2012, 2, 3927–3933 RSC.
- V. Rives, M. E. Pérez-Bernal, R. J. Ruano-Casero and I. Nebot-Díaz, J. Eur. Ceram. Soc., 2012, 32, 975–987 CrossRef CAS.
- M. Nocchetti, A. Donnadio, V. Ambrogi, P. Andreani, M. Bastianini, D. Pietrella and L. Latterini, J. Mater. Chem. B, 2013, 1, 2383–2393 RSC.
- W. F. Zhang, C. Ma, J. H. Fang, J. P. Cheng, X. B. Zhang, S. R. Dong and L. Zhang, RSC Adv., 2013, 3, 2483–2490 RSC.
- J. Sun, H. M. Liu, X. Chen, D. G. Evans and W. S. Yang, Nanoscale, 2013, 5, 7564–7571 RSC.
- J. B. Han, Y. B. Dou, M. Wei, G. E. David and X. Duan, RSC Adv., 2012, 2, 10488–10491 RSC.
- T. Hibino, Chem. Mater., 2004, 16, 5482–5488 CrossRef CAS.
- N. Iyi, S. Ishihara, Y. Kaneko and H. Yamada, Langmuir, 2013, 29, 2562–2571 CrossRef CAS.
- R. Z. Ma, Z. P. Liu, L. Li, N. Iyi and T. Sasaki, J. Mater. Chem., 2006, 16, 3809–3813 RSC.
- Q. L. Wu, A. Olafsen, Ø. B. Vistad, J. Roots and P. Norby, J. Mater. Chem., 2005, 15, 4695–4700 RSC.
- G. V. Manohara, D. A. Kunz, P. V. Kamath, W. Milius and J. Breu, Langmuir, 2010, 26, 15586–15591 CrossRef CAS.
- T. Hibino and W. Jones, J. Mater. Chem., 2001, 11, 1321–1323 RSC.
- J. Z. Wang, L. W. Zhao, H. M. Shi and J. He, Angew. Chem., Int. Ed., 2011, 50, 9171–9176 CrossRef CAS.
- A. Faour, C. Mousty, V. Prevot, B. Devouard, A. De Roy, P. Bordet, E. Elkaim and C. Taviot-Gueho, J. Phys. Chem. C, 2012, 116, 15646–15659 CAS.
- R. Z. Ma and T. Sasaki, Recent Pat. Nanotechnol., 2012, 6, 159–168 CrossRef CAS.
- R. Z. Ma, J. B. Liang, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2011, 133, 613–620 CrossRef CAS.
- R. Z. Ma, J. B. Liang, X. H. Liu and T. Sasaki, J. Am. Chem. Soc., 2012, 134, 19915–19921 CrossRef CAS.
- R. Z. Ma, M. Osada, L. F. Hu and T. Sasaki, Chem. Mater., 2010, 22, 6341–6346 CrossRef CAS.
- R. Z. Ma, K. Takada, K. Fukuda, N. Iyi, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2008, 47, 86–89 CrossRef CAS.
- J. B. Liang, R. Z. Ma, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, Chem. Mater., 2010, 22, 371–378 CrossRef CAS.
- T. Hibino and M. Kobayashi, J. Mater. Chem., 2005, 15, 653–656 RSC.
- C. A. Antonyraj, P. Koilraj and S. Kannan, Chem. Commun., 2010, 46, 1902–1904 RSC.
- M. Adachi-Pagano, C. Forano and J. P. Besse, Chem. Commun., 2000, 91–92 RSC.
- M. Singh, M. I. Ogden, G. M. Parkinson, C. E. Buckley and J. Connolly, J. Mater. Chem., 2004, 14, 871–874 RSC.
- Y. Zhao, W. D. Yang, Y. Xue, X. Wang and T. Lin, J. Mater. Chem., 2011, 21, 4869–4874 RSC.
- M. Jobbágy and A. E. Regazzoni, J. Colloid Interface Sci., 2004, 175, 345–348 CrossRef.
- V. V. Naik, T. N. Ramesh and S. Vasudevan, J. Phys. Chem. Lett., 2011, 2, 1193–1198 CrossRef CAS.
- D. Varade and K. Haraguchi, J. Mater. Chem., 2012, 22, 17649–17655 RSC.
- C. R. Gordijo, V. R. L. Constantino and D. D. Silva, J. Solid State Chem., 2007, 180, 1967–1976 CrossRef CAS.
- J. Cai and L. N. Zhang, Biomacromolecules, 2006, 7, 183–189 CrossRef CAS PubMed.
- J. Cai and L. N. Zhang, Macromol. Biosci., 2005, 5, 539–548 CrossRef CAS PubMed.
- B. Xiong, P. P. Zhao, P. Cai, L. N. Zhang, K. Hu and G. Z. Cheng, Cellulose, 2013, 20, 613–621 CrossRef CAS.
- J. Cai, L. N. Zhang, S. L. Liu, Y. T. Liu, X. J. Xu, X. M. Chen, B. Chu, X. Guo, J. Xu, H. Cheng, C. C. Han and S. Kuga, Macromolecules, 2008, 41, 9345–9351 CrossRef CAS.
- X. Z. Qin, A. Lu, J. Cai and L. N. Zhang, Carbohydr. Polym., 2013, 92, 1315–1320 CrossRef CAS PubMed.
- J. Cai, L. N. Zhang, J. P. Zhou, H. S. Qi, H. Chen, T. Kondo, X. M. Chen and B. Chu, Adv. Mater., 2007, 19, 821–825 CrossRef CAS.
- Q. N. Yang, X. Z. Qin and L. N. Zhang, Cellulose, 2011, 18, 681–688 CrossRef CAS.
- N. Iyi, K. Tamura and H. Yamada, J. Colloid Interface Sci., 2009, 340, 67–73 CrossRef CAS PubMed.
- S. Werner, V. W. H. Lau, S. Hug, V. Duppel, H. Clausen-Schaumann and B. V. Lotsch, Langmuir, 2013, 29, 9199–9207 CrossRef CAS PubMed.
- F. Leroux and J. P. Besse, Chem. Mater., 2001, 13, 3507–3515 CrossRef CAS.
- X. D. Lei, L. Yang, F. Z. Zhang and X. Duan, Chem. Eng. Sci., 2006, 61, 2730–2735 CrossRef CAS.
- X. Xu, F. Z. Zhang, S. L. Xu, J. He, L. Y. Wang, D. G. Evans and X. Duan, Chem. Commun., 2009, 46, 7533–7535 RSC.
| This journal is © The Royal Society of Chemistry 2014 |