Structural transition of VO2 (A) nanorods studied by vibrational spectroscopies†
Jiwei Houa,
Jianwu Zhang*a,
Zhongping Wangb,
Zengming Zhangb and
Zejun Dinga
aDepartment of Physics, Hefei National Laboratory for Physical Sciences at Microscale, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: zjw@ustc.edu.cn; Fax: +86-551-63600614; Tel: +86-551-63600614
bThe Centre of Physical Experiments, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 7th April 2014
Abstract
Single crystalline metastable phase VO2 (A) nanorods were successfully synthesized by using a hydrothermal method, and the evolution of vibrational modes of VO2 (A) nanorods has been observed in the vicinity of the structural phase transition. Fourier transform infrared (FT-IR) spectroscopy was employed to study the structural phase transition, and the abrupt changes of vibrational absorption bands at different temperatures showed a reversible phase transition. Raman spectrum measurement was performed to observe firstly the temperature-induced phase transition of VO2 (A) nanorods. The Raman modes near the phase transition temperature display the phonon softening behaviour.
Introduction
In recent years many low-dimensional nanomaterials, such as nanowires, nanorods, nanobelts and nanotubes, have become the focus of great interest due to their fundamental research importance and wide range of unique applications in fabrication of nanoscale devices,1–3 which exhibit novel physical and chemical properties compared with those of bulk materials. Vanadium dioxide (VO2) nanostructures have attracted increasing attention because of many promising properties for various potential applications in the nanometer regime.4–7 Vanadium dioxide is a representative binary compound having different polymorphs, including VO2 (M), VO2 (R), VO2 (B) and VO2 (A) and recently reported VO2 (C).8,9 Among these polymorphs, the rutile phase VO2 (R) is the most thermodynamic stable phase and has been attracting much attention for the reason that it has been observed a reversible metal–insulator phase transition, from a low temperature monoclinic phase VO2 (M) to a high temperature rutile phase VO2 (R), at around 68 °C.10 Accompanied with this phase transition, VO2 (R) shows a reversible abrupt changes in the electrical, magnetic and optical properties, especially for optical properties in the infrared region.11 Such properties make VO2 (R) potential applications in many fields, such as the gas sensor, optical switching devices, Mott field-effect transistors and the optical data storage media.12–14 Meanwhile, the structures, properties and potential applications of metastable VO2 (B) as a high potential candidate for the cathode materials in lithium-ion batteries have been widely studied. In addition, VO2 (B) as a precursor for preparing VO2 (R) has also been widely studied. We have investigated previously the monoclinic VO2 nanoparticles; the phase transition temperature was effectively controlled by the different W-doped concentrations.15 However, another metastable phase VO2 (A), which also exhibits a similar temperature-induced structural phase transition, has been paid less attention for the reason that this metastable phase is usually absent during the preparation of VO2 polymorph.The presence of metastable phase VO2 (A) was firstly reported by Théobald via a hydrothermal reaction of the V2O3–V2O5–H2O system.16 Several decades later, Oka et al. have prepared VO2 (A) by a hydrothermal method and studied the crystal structures of the low temperature and high temperature phases of VO2 (A).17 Subsequently, based on the crystal structure of VO2 (A), thermal stability, optical properties and electrical properties of VO2 (A) have been investigated.18,19 The study on the physical properties of VO2 (A) shows that VO2 (A) undergoes a reversible structural phase transition from its low temperature tetragonal phase to the high temperature body-center tetragonal phase (AH) at around 162 °C. The high temperature X-ray analysis showed that the phase transition of VO2 (A) is originated in the dissociation of the V4+–V4+ pairs along the c-axis.20 Meanwhile, a simple crystallographic slip mechanism was proposed to explain the phase transition process from VO2 (B) to VO2 (A).21 The Raman signal of VO2 (A) is very weak and thermal heating sensitive; even several mW of fine focused laser radiation is enough to induce the phase transition in VO2 (A). There were no reports on studying structural phase transition of VO2 (A) nanostructures by using vibrational spectroscopy, including Raman and FT-IR spectroscopy. In this work, we have greatly reduced the incident light intensity to avoid phase transition due to thermal heating of fine focused laser beam. The vibrational spectra of VO2 (A) were successfully observed during the phase transition.
To the best of our knowledge, vibrational spectroscopy had been used to study the structural phase transition of VO2 (M/R).22,23 In contrast to VO2 (M/R), metastable phase VO2 (A) has been comparatively attracted less attention, and there are no reports on the study of phase transition of VO2 (A) nanostructures by using Raman and FT-IR spectroscopies. In the present study, we have developed a facile way to prepare single crystalline VO2 (A) nanorods by a hydrothermal method; we report the investigation of the phase transition of VO2 (A) nanorods by using Raman scattering and FT-IR spectroscopy spectra. The evolution of vibrational modes of VO2 (A) nanorods is discussed for the temperatures near the phase transition.
Experimental
The single crystalline metastable phase VO2 (A) nanorods were synthesized by a hydrothermal method using VOSO4 as the raw material. In a typical procedure, a given amount of VOSO4 powder was dispersed into 80 ml of deionized water to form a clear blue solution after stirring for 30 minutes, and then the blue solution was transferred into a 100 ml Teflon-lined autoclave with a stainless steel shell at 220 °C for 24 h. When the reaction was finished, the autoclave was cooled naturally down to room temperature. Then the resulting brown slurry was collected by centrifugation, washed with distilled water and ethanol for several times, and finally dried in vacuum at 60 °C for 8 h.The crystal structure of obtained samples was recorded on a Shimadzu X-ray diffraction-7000 with Cu-Kα radiation. Morphology of the sample was observed with a field emission scanning electron microscope (SEM, Hitachi S-4800). The transmission electron microscopy (TEM) images, high resolution transmission electron microscopy (HRTEM) images and selected area electron diffraction (SAED) patterns were acquired on a JEOL-2010 transmission electron microscope at an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were performed on a VGESCALAB MKII X-ray photoelectron spectrometer with an excitation source of Mg-Kα, 1253.6 eV. Differential scanning calorimetery (DSC) was measured by a NETZSCH DSC 200 from 20 to 200 °C with the heating rate of 10 °C min−1. The FT-IR spectra of the samples, using pressed KBr tablets, were detected on a TENSOR-37 with an adapted heating controlled cell. The Raman spectra were recorded by using an integrated laser Raman system (LABRAMHR, Jobin Yvon) with a confocal microscope and a thermoelectrically cooled multichannel charge-coupled device (CCD) detection system; the spectral resolution was better than 1 cm−1. The line 514.5 nm of an argon laser was used as excitation source with a power less than 10 mW. All Raman spectra were measured in the backscattering geometry at the different temperatures.
Results and discussion
Fig. 1(b) presents a typical XRD pattern of the as-obtained product; all the diffraction peaks can be perfectly indexed to the metastable tetragonal crystalline phase of VO2 (A) (space group: P42/ncm, a = 8.434 Å, c = 7.681 Å), in agreement with the literature values (JCPDS no. 80-0690, a = 8.434 Å, c = 7.678 Å), whose plots are shown in Fig. 1(a). No characteristic peaks of any other phases and impurities are detected, indicating the as-obtained product with high purity. It is noteworthy that the stronger peaks belongs to the (hk0) family, suggesting that the VO2 (A) nanorods have a preferential growth direction along the [110], which is in agreement with HRTEM and SAED analysis (Fig. 3(d)).The surface composition of as-obtained sample was analyzed with XPS spectrum shown in Fig. 2. No peaks of elements other than C, O and V are observed on the survey spectrum. The peaks for O can be attributed to the VO2, O2, CO2 or H2O; the C1s peak is due to CO2 absorbed on the sample surface. According to the literature, two peaks at 516.2 and 523.8 eV in the high resolution spectrum (inset of Fig. 2) are attributed to the spin–orbit splitting of V2p3/2 and V2p1/2 and the intensity ratio is near 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is the characteristic of vanadium in the +4 oxidation state.24
1, which is the characteristic of vanadium in the +4 oxidation state.24
The other peaks at 631.0, 69.0 and 40.0 eV are attributed to V2s, V3s and V3p excitations, respectively. Consequently, the as-obtained sample can be determined as high pure tetragonal VO2 (A) on basis of the results of XRD and XPS measurements.
The detailed morphology and microstructure of samples were investigated by SEM and TEM analysis; the typical SEM images of VO2 (A) at different magnifications are shown in Fig. 3. VO2 (A) nanorods with the rod-like morphology and in length of several tens of micrometers are clearly displayed. The average diameter of nanorods is about 200 nm in Fig. 3(b). The diameter statistics of VO2 (A) nanorods have been given (Fig. S1, ESI†). A HRTEM image of a single nanorod and a SAED pattern (the lower inset in Fig. 3(d)) obtained from the same single nanorod are shown in Fig. 3(c) and (d), respectively. The lattice fringes are clearly exhibited and the 0.536 nm of neighboring fringe spacing matches well the separation between (110) lattice planes. The corresponding SAED pattern further demonstrates that the nanorod is single crystalline and growth direction is along the [110] direction.
In order to further investigate the structure of VO2 (A) nanorods in situ variable temperature X-ray diffraction measurements were performed on a Pt substrate in the temperature range from 25 to 220 °C. The evolution of the XRD patterns of VO2 (A) nanorods as a function of temperature is exhibited in Fig. 4.
The XRD pattern at lower temperature of 160 °C can be readily indexed into low-temperature VO2 (A) phase, while that above 170 °C is assigned to a high-temperature phase VO2 (AH). The shape of the XRD pattern has almost no obvious change with temperature increasing except the disappearance of the (211), (310), (311), (420) and (114) diffraction peaks above phase transition temperature, indicating that the crystal structure does not change drastically. The disappearance of the diffraction peaks above the phase transition temperature, indicating that there are two different crystal structures of VO2 (A) between 160 and 170 °C. The XRD pattern at 25 °C is reproduced upon cooling down from 220 °C, the reversible change of the diffraction peaks confirms a reversible phase transition between initial phase and high temperature phase during heating and cooling processes.
The evolution of the lattice parameters associated with the temperature has been calculated and a slight increase of lattice parameter, a- and b-values, is observed from 8.434 Å to 8.472 Å (heat-treated at 220 °C) and an obvious change of the lattice parameter c-value is observed from 7.684 Å to 3.821 Å (heat-treated 220 °C). The results of lattice parameters are consistent with previously reported.21 The a/c ratio in the tetragonal phase is increased from 1.098 to 2.216 with increasing temperature.
Phase transition in VO2 system using DSC analysis has been studied widely especially for monoclinic phase VO2 (M/R), for which the first order metal–insulator phase transition occurs at 68 °C.25,26 In order to investigate the phase transition properties of VO2 nanorods, the DSC analysis was performed. There is an endothermic peak at 169 °C and an exothermic peak at 133 °C, as show in Fig. 5, in DCS curve. The DSC data show that the structure phase transition temperature of VO2 (A) nanorods exist the obviously thermal hysteresis phenomenon. The endothermic peak at 169 °C can be assigned to conversion of the initial tetragonal into a high-temperature phase during heating process. Meanwhile, an exothermic peak at 133 °C can be attributed to the crystallization of the VO2 (AH) into the VO2 (A) form upon cooling. The wide hysteresis loop of VO2 (A) nanorods is considered to be due to the scaling to nanoscale domain, as observed in VO2 (M/R) nanostructures.27 The endothermic peak was slightly higher than 162 °C reported in the literatures due to the size effect and morphology of the nanostructure. Meanwhile, effects of size and morphology of monoclinic VO2 metal–insulator phase transition has been extensively studied.28
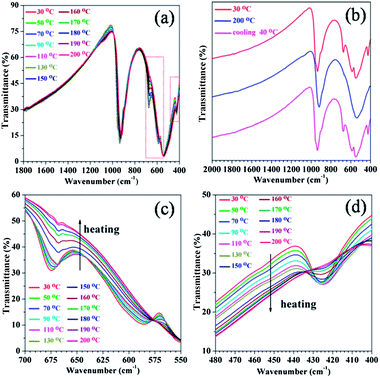
FT-IR spectroscopy is an excellent analytical method, which has been widely utilized to study the metal–insulator transition of VO2 (M/R) nanostructures.15,29 In order to observe the evolution behavior of vibrational modes of VO2 (A) nanorods in the phase transition, an in situ variable-temperature IR spectroscopy was performed to further study the vibration bands. A series of FT-IR spectra of the as-obtained VO2 (A) nanorods under varied temperatures are presented in Fig. 6. At 25 °C, the IR spectra of single domain VO2 (A) nanorods clearly display the existence of various V–O vibrations. According to the reported IR spectra of vanadium dioxide, the vibration band at 932 cm−1 is assigned to the stretching of V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, while the bands at 548 and 426 cm−1 are attributed to the long-rang stretching of V–O–V vibration modes. The bands at 980 cm−1 or 1020 cm−1 are absent, which are the characteristic peaks of V3+ or V5+,30,31 respectively, which is consistent with our XRD results.
O bonds, while the bands at 548 and 426 cm−1 are attributed to the long-rang stretching of V–O–V vibration modes. The bands at 980 cm−1 or 1020 cm−1 are absent, which are the characteristic peaks of V3+ or V5+,30,31 respectively, which is consistent with our XRD results.
The temperature-dependent FT-IR spectra of the VO2 (A) nanorods were investigated the phase transition. The IR spectra in Fig. 6 display various V–O vibrations, and the vibration bands at 935 and 547 cm−1 gradually shifted to lower wavenumbers with increasing temperature. The slight red shift of vibration bands indicates a phonon mode soften behavior. Fig. 6(c) and (d) show that the vibratory absorption bands at 670, 580 and 426 cm−1 become gradually weaken and disappear with increasing temperature, while these bands reappear with lowering down temperature in Fig. 6(b). This optical property has a potential application in optical switching devices and the similar optical property was observed in VO2 (M/R).15,26,29 The FT-IR spectrum is usually interpreted as that the observed bands are related to the electromagnetic resonance between the incident photons and variation of chemical bond polarization associated with a specific vibration mode. The V4+–V4+ bonds in low temperature VO2 (A) phase with a distance of 2.769 Å is dissociated in high temperature VO2 (A) phase with a distance of 3.079 Å reported by Oka.17 At low temperature the electrons involving in the V4+–V4+ bonds between VO6 octahedra are localized, whereas these electrons are delocalized at high temperature. This delocalization involves a screening effect for the incident photons and leads to the vibration bands disappeared during increasing temperature.
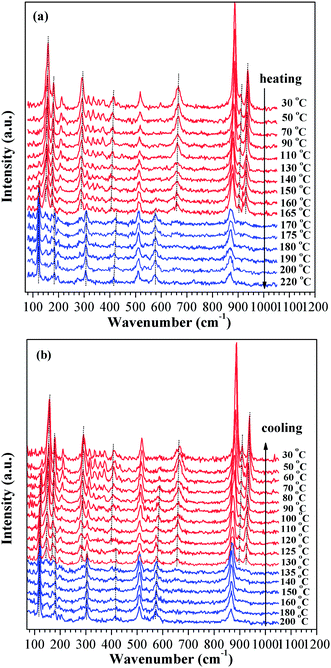
In order to study the temperature induced phase transition of VO2 (A) nanorods, an in situ Raman scattering measurement was performed under different temperatures. We reduced the incident laser intensity by about two orders to be roughly 0.1 mW to ensure that thermal heating due to focusing laser would not result in a phase transition. The evolution of Raman modes for VO2 (A) nanorods under various temperatures is presented in Fig. 7 ((a) heating, (b) cooling). Ten Raman active modes are observed in the Raman spectrum for 30 °C at 159, 180, 212, 291, 413, 518, 666, 887, 910 and 938 cm−1, which are different with the known Raman modes of VO2 (M/R).32
For temperatures up to 165 °C, all the Raman modes are assigned to the low temperature phase. At 170 °C, some new Raman modes appear at 123, 184, 308, 422 and 578 cm−1, which are the characteristic peaks of high temperature phase of VO2 (A); the intensity of these new Raman modes become stronger and shaper with increasing temperature. Meanwhile, the Raman modes of the low temperature phase with frequencies at 159, 180, 291, 416, 666, 910 and 938 cm−1 abruptly disappear. The discontinuities of Raman modes with temperature are clearly observed between 165 and 170 °C, indicating a temperature-induced phase transition occurred between 165 and 170 °C. The Raman vibrational modes of low temperature phase are fully restored during the temperature cooling down to ambient condition. The cooling process in the variable temperature Raman measurement show the reversible structure transition between 130 and 135 °C in Fig. 7(b). The results are well in agreement with the variable temperature X-ray diffraction and DSC data.
The evolution of the Raman modes appears to be almost linear until the temperature up to 165 °C. The relationship between Raman peaks and temperature is fitted linearly with the slope called red shift coefficient α. The red shift coefficients determined from the fits are: α = 0.059, 0.044, 0.052, 0.067, 0.074 and 0.089 cm−1 K−1 for 160, 180, 413, 887, 910 and 938 cm−1, respectively. It can be seen that the Raman modes exhibit an obvious red shift, indicating the electron interaction with the lattice is enhanced during the increasing the crystal temperature. An evidence of phonon softening behavior near the phase transition of VO2 (A) was observed. The phonon soften behavior indicates that the driving mechanism of the phase transition of VO2 (A) involve electron–phonon interaction. The electron–phonon interaction has been extensively studied using the Raman spectrum in the metal–insulator transition of VO2 (M/R); the experimental and theoretical studies have confirmed that the electron–phonon interaction plays a crucial role in the metal–insulator transition of VO2 (M/R).33,34
Conclusions
In summary, VO2 (A) nanorods were successfully fabricated through a facile and environmental friendly hydrothermal method without using any surfactants or templates. The as-obtained nanorods have diameters of 100–300 nm and length of several tens of micrometers. A reversible structural phase transition around 160–170 °C has been observed through the variable-temperature X-ray diffraction and Raman spectroscopy measurement. An evidence of phonon softening behavior near the temperature of phase transition was observed through the variable-temperature vibrational spectrum. The electron–phonon interaction plays a role in the phase transition of VO2 (A) nanorods.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 11174265, 11274288 and 21002097) and “973” projects (no. 2011CB932801, 2012CB933702 and 2009CB939901).Notes and references
- C. R. Xiong, A. E. Aliev, B. Gnade and K. J. Balkus, ACS Nano, 2008, 2, 294 CrossRef PubMed.
- Z. Gui, R. Fan, W. Q. Mo, X. H. Chen, L. Yang and W. C. Fan, Chem. Mater., 2002, 14, 5055 CrossRef.
- C. Nethravathi, B. Viswanath, J. Michael and M. Rajamath, Carbon, 2012, 50, 4841 CrossRef PubMed.
- C. H. Chen, R. F. Wang, L. Shang and C. F. Guo, Appl. Phys. Lett., 2008, 93, 171101 CrossRef PubMed.
- J. M. Wu and L. B. Liou, J. Mater. Chem., 2011, 21, 5503 Search PubMed.
- X. H. Rui, D. H. Sim, C. Xu, W. L. Liu, H. T. Tan, K. M. Wong, H. H. Hng, T. M. Lim and Q. Y. Yan, RSC Adv., 2012, 2, 1178 RSC.
- L. Dai, Y. F. Gao, C. X. Cao, Z. Chen, H. J. Luo, M. Kanehira, J. Jin and Y. Liu, RSC Adv., 2012, 2, 5268 Search PubMed.
- C. Leroux, G. Nihoul and G. V. Tendeloo, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 5111 CrossRef CAS.
- D. Hangrman, J. Zubieta, C. J. Warren and L. M. Meyer, J. Solid State Chem., 1998, 138, 178 CrossRef.
- F. J. Morin, Phys. Rev. Lett., 1959, 3, 34 CrossRef CAS.
- B. S. Guition, Q. Gu, L. A. Prieto, M. S. Gudiksn and H. Park, J. Am. Chem. Soc., 2005, 127, 499 Search PubMed.
- E. Strelcov, Y. Lilach and A. Kolmakov, Nano Lett., 2009, 9, 2324 CrossRef PubMed.
- S. Bonora, U. Bortolozzo, S. Residori, R. Balu and P. V. Ashrit, Opt. Lett., 2010, 35, 104 CrossRef PubMed.
- H. T. Kim, B. G. Chae, D. H. Youn, S. L. Maeng, G. Kim, K. Y. Kang and Y. S. Lim, New J. Phys., 2004, 6, 52 CrossRef.
- J. W. Hou, J. W. Zhang, Z. P. Wang, Z. M. Zhang and Z. J. Ding, J. Nanosci. Nanotechnol., 2013, 13, 1546 CrossRef PubMed.
- F. Théobald, J. Less-Common Met., 1977, 53, 55 CrossRef.
- Y. Oka, S. Sato, T. Yao and N. Yamamoto, J. Solid State Chem., 1998, 141, 595 Search PubMed.
- M. Li, F. G. Kong, Y. X. Zhang, L. Chen, W. W. Yan and G. H. Li, Dalton Trans., 2011, 40, 10964 Search PubMed.
- S. D. Zhang, B. Shang, J. L. Yang, W. S. Yan, S. Q. Wei and Y. Xie, Phys. Chem. Chem. Phys., 2011, 13, 15880 Search PubMed.
- T. Yao, Y. Oka and N. Yamamoto, J. Solid State Chem., 1994, 112, 197 CrossRef.
- J. Galy, J. Solid State Chem., 1999, 148, 226 CrossRef.
- E. U. Donev, R. Lopez, L. C. Feldman and R. F. Haglund, Nano Lett., 2009, 9, 703 Search PubMed.
- S. X. Zhang, J. Y. Chou and L. J. Lauhon, Nano Lett., 2009, 9, 4528 Search PubMed.
- J. Mendialdua, R. Casanova and Y. Barbaux, J. Electron Spectrosc. Relat. Phenom., 1995, 71, 252 CrossRef.
- C. X. Cao, Y. F. Gao and H. J. Luo, J. Phys. Chem. C, 2008, 112, 11813 Search PubMed.
- Z. F. Peng, W. Jiang and H. Liu, J. Phys. Chem. C, 2007, 111, 1121 Search PubMed.
- K. W. Lee, J. J. Kweon, C. E. Lee, A. Gedanken and R. Ganesan, Appl. Phys. Lett., 2010, 96, 243111 CrossRef PubMed.
- J. M. Baik, M. H. Kim, C. Larson, A. M. Wodtke and M. Martin, J. Phys. Chem. C, 2008, 112, 13330 Search PubMed.
- Y. F. Sun, S. S. Jiang, W. T. Bi, X. G. Tan, C. Z. Wu, S. Q. Wei and Y. Xie, Nanoscale, 2011, 3, 4394 RSC.
- J. C. Valmalette and J. R. Gavarri, Mater. Sci. Eng., B, 1999, 54, 168 CrossRef.
- I. L. Botto, M. B. Vassallo, E. J. Baran and G. Minelli, Mater. Chem. Phys., 1997, 50, 269 CrossRef.
- P. Schible, Physica B, 2002, 316, 601 Search PubMed.
- S. Kim, K. Kim, C. J. Kang and B. I. Min, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 195106 CrossRef.
- J. M. Atkin, S. Berweger, E. K. Chavez and M. B. Raschke, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 020101 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00585f |
| This journal is © The Royal Society of Chemistry 2014 |