Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High internal phase emulsion gels (HIPE-gels) prepared using food-grade components†
Ashok R. Patel*a,
Ysamar Rodriguezb,
Ans Lesafferb and
Koen Dewettincka
aVandemoortele Centre ‘Lipid Science & Technology’, Lab. of Food Tech. & Engg., Faculty of Bioscience Engg., Ghent University, Belgium. E-mail: Patel.Ashok@Ugent.be; Fax: +32 9264 6218; Tel: +32 9264 6209
bVandemoortele R & D Centre, Prins Albertlaan 79, 8870 Izegem, Belgium
First published on 7th April 2014
Abstract
We report a new approach of using dispersed water phase gelation as a mode to create oil continuous emulsion gels. The low temperature gelation property of synergistic hydrocolloid combinations was exploited to develop elastic soft solids using only food-grade components.
Many of the regularly consumed food products are emulsion-based soft solids that display gel-like behaviour (structured emulsions).1,2 These viscoelastic products can be broadly categorized into three main classes including: (i) water continuous emulsions structured using proteins and/or polysaccharides that act as emulsifiers as well as thickening or gelling agents (e.g. cheese, yogurt, milk-based deserts etc.); (ii) highly concentrated water continuous emulsions where the rheological structuring comes from the close crowding of dispersed oil droplets at high packing fractions (such as mayonnaise, sauce etc.) and (iii) oil continuous emulsions structured using network of fine solid fat crystals (e.g. margarine, butter and spreads).3–5
While a lot of research has been conducted on studying and identifying ways to structure the water continuous emulsions, the exploration of alternate ways to structure oil continuous emulsions has been lagging far behind with most commercial products still relying on the use of solid fats for structure creation.5 In wake of the recent reports on health concerns relating to the consumption of saturated and trans-fats, some research has emerged in last few years where structured oil continuous emulsions have been prepared in absence of solid fats via oleogelation.6–11 However, the oleogelators used in these studies are either not suitable for food-grade applications (12-hydroxy stearic acid and waxes)12–16 or are rather expensive (phytosterols and oryzanol) to be considered for use in routine food products.17 There are some interesting non-food related research results where high internal phase emulsion (HIPE) organogels were achieved through the use of low molecular weight gelators and functionalized polymers (both of which requires a significant synthetic efforts and are non-food grade).18,19
In this paper, we report for the very first time a new approach to create structured soft solids based on gelation of oil continuous HIPE. The gelling of the emulsion was achieved through the use of food-grade hydrocolloids dispersed in the internal phase that constituted major volume of the emulsion (φwater > close packing volume). Galactomannans (natural polymers made up of galactose and mannose subunits) are known to show synergistic interactions with other food-grade hydrocolloids like carrageenan (CAR) and xanthan gum (XAN). In particular, locust bean gum (LBG), a galactomannan with a higher mannose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactose ratio (∼4
galactose ratio (∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
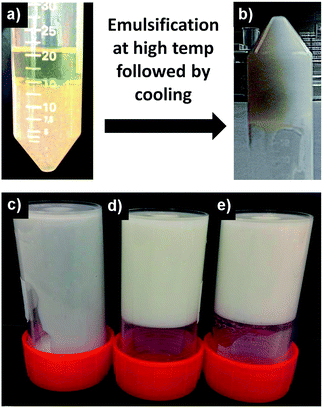
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displays a strong interaction with other non-gelling hydrocolloids leading to the formation of gel at a low total polymer concentration.20 In the current work, the synergistic gelling property of these hydrocolloid combinations was exploited to generate oil continuous HIPE gel by first finely dispersing hydrocolloid solution in oil continuous medium through emulsification at high temperature (>70 °C) followed by cooling to room temperature to trigger water droplet gelation (Fig. 1a).
1) displays a strong interaction with other non-gelling hydrocolloids leading to the formation of gel at a low total polymer concentration.20 In the current work, the synergistic gelling property of these hydrocolloid combinations was exploited to generate oil continuous HIPE gel by first finely dispersing hydrocolloid solution in oil continuous medium through emulsification at high temperature (>70 °C) followed by cooling to room temperature to trigger water droplet gelation (Fig. 1a).
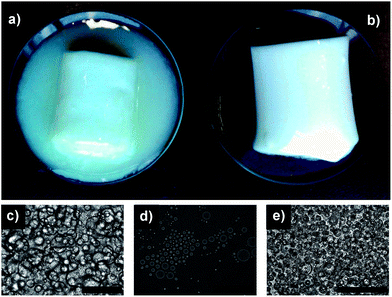
Due to the high phase volume, the gelled droplets provided a structural framework that held the emulsion together, resulting in a self-standing elastic gel-like structure. The effect of phase volume on the structuring is evident from Fig. 1b. At lower phase volumes (φwater = 0.25), the formed emulsion was fluid in nature and did not show any gelation. At higher phase volumes (φwater = 0.5 and 0.75), gelation of both emulsions was evident. However, emulsion with φwater = 0.5 showed physical instability overnight, leading to a distinct separation into a gel phase and a fluid phase (Fig. 2a). The optical microscopy images shown in Fig. 2c & d indicate that the emulsion in both the gel and separated fluid phase was stable with higher droplet population in close packing constituting the gelled phase and a dilute emulsion making up the separated fluid phase. In both cases, the emulsion did not show any coalescence. The emulsion with φwater = 0.75 on the other hand, gave an elastic self-standing gel (Fig. 2b) which was stable for over 3 months without showing any separation or droplet size increase. The tightly packed, gelled water droplets were seen under the microscope (Fig. 2e) suggesting that the close packing of gelled emulsion droplets is necessary to build a framework to support the 3D gel structure that physically traps oil phase in the inter-droplet sites lined by crowded interfaces.
Initial emulsification trials without hydrocolloids were carried out using commonly used food emulsifiers – monoglycerides (consisting of a fatty acid linked to glycerol backbone).21,22 However, due to their limited surface activity, the emulsions formed using monoglycerides even at concentrations as high as 5% wt were unstable and showed phase separation on cooling (Fig. S1a†). On the other hand, stable HIPE at φwater = 0.75 (with a mean droplet radius of 4.39 μm) was obtained by using polyglycerol polyricinoleate (PGPR) at a low concentration of 0.4% wt (Fig. S1b†). Unlike monoglycerides, PGPRs are polymeric surfactants (fatty acid esters of polyglycerol)23 with strong surface activity22 used for specialized food formulations like chocolate, low fat spreads and bakery products.24 The HIPE gels were formed by incorporating hydrocolloids (a combination of LBG:CAR and LBG:XAN) in the water phase. Though the hydrocolloids used are non-surface active, the HIPE gels had lower average droplet sizes (3.5 and 2.76 μm for LBG:CAR and LBG:XAN respectively) then HIPE emulsion (Fig. S2†). The probable reason for this decrease in the water droplet size could be due to the increase in the viscosity of the internal phase due to the incorporation of hydrocolloids. The decrease in the droplet size with the increase in the viscosity of dispersed phase could be explained by the fact that under the shear flow, the high viscosity droplets can resist disruption longer and thus undergo higher deformation before breaking down into smaller droplets.22,25 LBG:CAR and LBG:XAN are well-studied synergistic combinations used as formulation aid in various food products.26–28 The exact mechanism of these synergistic interactions are still unclear but they are utilized to obtain gels in combination with non-gelling hydrocolloids.28,29 The gels formed by these hydrocolloid combinations are low temperature setting and thermo reversible.20,30 The thermo reversible property of these hydrocolloid combinations was used to form HIPE gels by emulsifying the melted gel with oil at high temperature followed by cooling down to the room temperature in order to trigger gel formation of the internal phase.
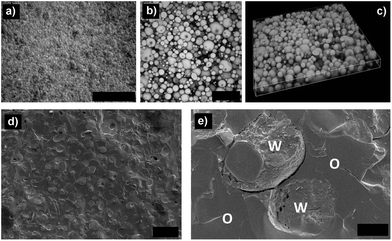
The microstructure of HIPE gel was studied using a range of microscopy (optical, confocal and cryo-SEM) techniques (Fig. 3). Visualization of emulsion under confocal microscope confirmed the oil-continuous nature of emulsion with dispersed gelled water droplets closely packed together (Fig. 3b & c). The deformation of droplet shapes indicates close packing structure of the emulsion which forms the main framework that supports the 3D gel structure. The gelled water droplets are further seen in the cryo-SEM images of freeze-fractured HIPE gel (Fig. 3c & d). The HIPE gels were stable over 3 months of storage as indicated by an absence of “oiling-off”, no coalescence and uniformity of average droplet size (Fig. S3†).
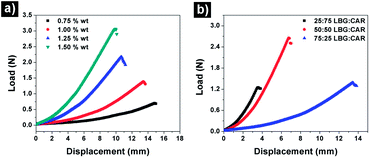
Food emulsion gels have interesting viscoelastic properties which are usually evaluated by studying their large deformation fracture properties and yielding behaviour.1,31–33 The HIPE gels were subjected to uni-axial compression testing to gain more information about the structural arrangement of these systems. Both the total polymer concentration as well as polymer proportions in the combinations had an effect on the fracture properties of HIPE gels. The increase in the polymer concentration resulted in a decrease of fracture strain as indicated by a decrease in the displacement values (l*) at fracture point or point of permanent deformation (Fig. 4 & Table S1†). The modulus of elasticity (Young's modulus, Ey) is a measure of the stiffness (rigidity) of a sample or in other words, the extent to which a sample resists deformation to an applied force.33,34 The values of Ey is calculated from the slope of the linear portion of load–displacement curve where the load is below elastic limit and the sample still obeys Hooke's law (i.e. load is proportional to extension of sample).34 On the other hand, critical force (F*) is a measure of the strength of a sample i.e. the ability to withstand an applied stress without failure.35 In terms of gels, a higher Ey indicates a stiffer gel that can be stretched only to a certain extent before it breaks and a higher fracture force indicates the higher gel strength or gel hardness.36,37 A desirable food emulsion gel needs to have a reasonable strength (intermediate to high F*) but at the same time a lower Ey and a higher fracture strain (higher displacement at fracture point, l*) so that it is stretchable (and consequently spreadable) over a larger surface before it undergoes plastic deformation. Understandably, the increase in the total polymer concentration led to an increase in the values of F* and Ey while showing a decrease in the values of l*. The HIPE gel prepared at a total polymer concentration of 0.75% wt showed lowest Ey (4.62 N m−2) but was very weak as indicated from a very low value of F* (0.69 N). On the other hand, samples made at 1.25% and 1.5% wt polymer concentration showed good gel strength (2.17 and 3.07 N respectively) but were very rigid (Ey = 15.78 and 23.23 N m−2 for 1.25 and 1.5% wt respectively) and showed a lower spreadability (l* = 10.67 and 9.92 mm for 1.25 and 1.5% wt respectively). A total polymer concentration of 1% wt showed a reasonable gel strength (F* > 1 N) as well as a high fracture strain (l* = 13.42 mm) and hence, it was selected for further fracture property evaluation by varying the proportion of hydrocolloids in the emulsion gels (Fig. 4b). As usually seen in LBG:CAR gels,38 the increase in the LBG proportion led to an increased elasticity of emulsion gels as indicated from the increase in the fracture strain (l* = 3.59 mm for LBG:CAR, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 to l* = 13.42 mm for LBG:CAR, 75
75 to l* = 13.42 mm for LBG:CAR, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). On the other hand, a stiff gel with high gel strength (Ey = 19.18 N m−2 and F* = 2.65 N) was obtained at LBG:CAR proportion of 50
25). On the other hand, a stiff gel with high gel strength (Ey = 19.18 N m−2 and F* = 2.65 N) was obtained at LBG:CAR proportion of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The increased gel strength at a 50
50. The increased gel strength at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio can be explained based on the synergistic interactions between carrageenan and LBG which are strongest at proportions of LBG:CAR between 60
50 ratio can be explained based on the synergistic interactions between carrageenan and LBG which are strongest at proportions of LBG:CAR between 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 40
40 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60,39 the interactions are due to the involvement of the smooth un-branched segments of D-mannose backbone in H-bonding.26,40 In our study, LBG:CAR ratio of 75
60,39 the interactions are due to the involvement of the smooth un-branched segments of D-mannose backbone in H-bonding.26,40 In our study, LBG:CAR ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 was selected as a balance between required elasticity (higher l*) and intermediate stiffness (lower Ey).
25 was selected as a balance between required elasticity (higher l*) and intermediate stiffness (lower Ey).
The HIPE gels prepared using hydrocolloid combinations of LBG:CAR and LBG:XAN at a total polymer concentration of 1% wt (and LBG proportion of 75% in the hydrocolloid combinations) were further compared using relaxation test and 2-cycle compression tests or texture profile analysis (TPA). The comparative relaxation curves (Fig. 5a) confirms the viscoelastic behaviour of these gels where a prominent stress relaxation was observed under a constant strain. In other words, the stress required to maintain the constant strain decreased with the increase in time.41 Depending on the type of gels, such behaviour is explained via two possible mechanisms: (a) diffusion-driven relaxation due to the transport of water in and out of the gel network (seen in chemical gels) or (b) network rearrangement based weakening of physical linkages (physical gels).42 In our case, since there was no fluid release from the gels at the end of these tests, the stress relaxation can be speculated to be due to the weakening of the network of closely packed gelled water droplets resulting in the progressive loss of energy storage capability of the structural framework. Additionally, the deformation of gelled water droplets will also have a significant role to play. Even though we are working at a high dispersed phase volume fractions, the microscopy evaluation show that the droplets are still spherical and haven't undergone a maximum deformation (polygonal shape). Thus, under strain which is lower than the fracture strain, the spherical droplets may undergo further deformation resulting in the restructuring of the network and contributing to the stress dissipation. It can also been seen that the peak load for HIPE gel prepared using LBG:CAR was much higher than LBG:XAN indicating a clear difference in the strength of these HIPE gels. The difference in the properties of these gels were further quantified using parameters (Table 1) obtained from TPA curves (Fig. 5b & c). The HIPE gel prepared using LBG:XAN had a much lower firmness (0.57 N as compared to 10.79 N for LBG:CAR) and showed a prominent brittleness (fracturability = 0.29 N) as indicated by red arrow in Fig. 5c. The comparatively higher elasticity of LBG:CAR can also be seen from the higher value of chewiness (energy required for breakdown of the gel over consecutive cycles). Moreover, LBG:XAN gel was sticky in nature as indicated by an adhesiveness value of 1.7 × 10−3 N m−2 (adhesiveness is a measure of negative work done on the probe during its upward movement).43 Hence, it is clear from these results that there was a distinct difference in the large deformation fracture properties of HIPE gels prepared by combining LBG with two different hydrocolloids (CAR and XAN).
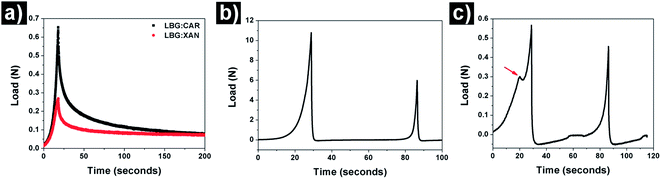 | ||
| Fig. 5 (a) Comparative curves obtained from relaxation tests done on HIPE gels made using LBG:CAR and LBG:XAN; (b & c) TPA curves for HIPE gels made using LBG:CAR and LBG:XAN respectively. | ||
| Parameters | LBG:CAR | LBG:XAN |
|---|---|---|
| a The differences were statistically non-significant (p > 0.05). | ||
| Firmness (N) | 10.79 ± 0.61 | 0.57 ± 0.01 |
| Cohesivenessa | 0.19 ± 0.02 | 0.23 ± 0.01 |
| Springiness indexa | 0.48 ± 0.03 | 0.44 ± 0.03 |
| Adhesiveness (N m−2) | — | 1.7 × 10−3 |
| Fracturability (N) | — | 0.29 ± 0.001 |
| Resiliencea | 0.07 ± 0.003 | 0.04 ± 0.002 |
| Chewiness (N m−2) | 0.98 ± 0.06 | 0.06 ± 0.01 |
Conclusions
In conclusion, we successfully demonstrated a facile approach of making oil continuous HIPE gels using only common food-grade components (hydrocolloids and a food emulsifier). To develop such systems, two important considerations need to be taken in to account: first is the creation of a stable oil continuous HIPE followed by selecting appropriate hydrocolloids for gelling the internal water droplet phase. The hydrocolloids to be used for these systems should show a thermoreversible, low temperature gelation. A combination of hydrocolloids is more appropriate as it gives us wider options to influence the properties of formed gels based on the polymer proportions. It is also necessary to use hydrocolloids that are non-surface active in order to prevent the inversion of the systems into water continuous gel.We believe that the possibility of structuring oil continuous gel systems without the use of any solid fats could be of significant importance to food product development especially for lowering of fat levels.
Acknowledgements
This research is supported by Marie Curie Career Integration Grant within the 7th European Community Framework Programme.Notes and references
- E. Dickinson, Food Hydrocolloids, 2012, 28, 224–241 CrossRef CAS.
- V. B. Tolstoguzov and E. E. Braudo, J. Texture Stud., 1983, 14, 183–212 CrossRef CAS.
- M. M. Robins and P. J. Wilde, in Encyclopedia of Food Sciences and Nutrition, ed. B. Caballero, Academic Press, Oxford, 2nd edn, 2003, pp. 1517–1524 Search PubMed.
- E. Dickinson, Curr. Opin. Colloid Interface Sci., 2010, 15, 40–49 CrossRef CAS.
- A. Bot, E. Flöter, H. P. Karbstein-Schuchmann and H. S. Ribeiro, in Product Design and Engineering, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, pp. 315–343 Search PubMed.
- A. Bot and W. M. Agterof, J. Am. Oil Chem. Soc., 2006, 83, 513–521 CrossRef CAS.
- A. Bot and E. Floter, in Edible Oleogels: Structure and Health Implications, ed. A. G. Marangoni and N. Garti, AOCS Press, Urbana, IL, USA, 2011, pp. 49–79 Search PubMed.
- L. S. K. Dassanayake, D. R. Kodali and S. Ueno, Curr. Opin. Colloid Interface Sci., 2011, 16, 432–439 CrossRef CAS.
- T. Dey, D. A. Kim and A. G. Marangoni, in Edible Oleogels: Structure and Health Implications, ed. A. G. Marangoni and N. Garti, AOCS Press, Urbana, Illinois, 2011, pp. 295–312 Search PubMed.
- A. Marangoni, J. Am. Oil Chem. Soc., 2012, 89, 749–780 CrossRef CAS.
- A. G. Marangoni, N. Acevedo, F. Maleky, E. Co, F. Peyronel, G. Mazzanti, B. Quinn and D. Pink, Soft Matter, 2012, 8, 1275–1300 RSC.
- Edible oleogels: Structure and health implications, ed. A. G. Marangoni and N. Garti, AOCS Press, Urbana, Illinois, 2011 Search PubMed.
- A. R. Patel, D. Schatteman, W. H. De Vos, A. Lesaffer and K. Dewettinck, J. Colloid Interface Sci., 2013, 411, 114–121 CrossRef CAS.
- A. R. Patel, D. Schatteman, W. H. D. Vos and K. Dewettinck, RSC Adv., 2013, 3, 5324–5327 RSC.
- J. Toro-Vazquez, J. Morales-Rueda, V. A. Mallia and R. Weiss, Food Biophys., 2010, 5, 193–202 CrossRef.
- A. R. Patel, P. S. Rajarethinem, A. Gredowska, O. Turhan, A. Lesaffer, W. H. De Vos, D. Van de Walle and K. Dewettinck, Food Funct., 2014, 5, 645–652 CAS.
- H. Sawalha, R. den Adel, P. Venema, A. Bot, E. Flöter and E. van der Linden, J. Agric. Food Chem., 2012, 60, 3462–3470 CrossRef CAS.
- Y. Chen, N. Ballard, F. Gayet and S. A. F. Bon, Chem. Commun., 2012, 48, 1117–1119 RSC.
- T. Zhang and Q. Guo, Chem. Commun., 2013, 49, 11803–11805 RSC.
- Functional Properties of Food Macromolecules, ed. S. E. Hill, D. A. Ledward and J. R. Mitchell, Aspen Publishers Inc., Maryland, USA, 1998 Search PubMed.
- A. Sein, J. A. Verheij and W. G. M. Agterof, J. Colloid Interface Sci., 2002, 249, 412–422 CrossRef CAS.
- Encylcopedic Handbook of Emulsion Technology, ed. J. Sjoblom, Marcel Dekker Inc., New York, USA, 2001 Search PubMed.
- N. Garti, A. Aserin and B. Zaidman, J. Am. Oil Chem. Soc., 1981, 58, 878–883 CrossRef CAS.
- Food Emulsifiers and Their Applications, ed. G. N. Hasenhuetll and R. W. Hartel, Chapman & Hall, New York, USA, 1997 Search PubMed.
- S. R. Derkach, International Review of Chemical Engineering, 2010, 2, 465–472 Search PubMed.
- M. Tako, Z.-Q. Qi, E. Yoza and S. Toyama, Food Res. Int., 1998, 31, 543–548 CrossRef CAS.
- R. O. Mannion, C. D. Melia, B. Launay, G. Cuvelier, S. E. Hill, S. E. Harding and J. R. Mitchell, Carbohydr. Polym., 1992, 19, 91–97 CrossRef CAS.
- Food Polysaccharides and Their Applications, ed. A. M. Stephan and G. O. Phillips, CRC Press, FL, USA, 2006 Search PubMed.
- D. F. Zhan, M. J. Ridout, G. J. Brownsey and V. J. Morris, Carbohydr. Polym., 1993, 21, 53–58 CrossRef CAS.
- M. Tako, T. Teruya, Y. Tamaki and K. Ohkawa, Colloid Polym. Sci., 2010, 288, 1161–1166 CAS.
- G. Sala, R. A. de Wijk, F. van de Velde and G. A. van Aken, Food Hydrocolloids, 2008, 22, 353–363 CrossRef CAS.
- G. Sala, F. van de Velde, M. A. Cohen Stuart and G. A. van Aken, Food Hydrocolloids, 2007, 21, 977–985 CrossRef CAS.
- Texture in Food, ed. D. Kilcast, Woodhead Publishing Ltd & CRC Press, Cambridge, UK, 2004 Search PubMed.
- Food Texture: Instrumental and Sensory Measurement, ed. H. R. Moskowitz, Marcel Dekker Inc., New York, USA, 1987 Search PubMed.
- D. D. Hamann, J. Zhang, C. R. Daubert, E. A. Foegeding and K. C. Diehl, J. Texture Stud., 2006, 37, 620–639 CrossRef.
- G. Sala, G. A. Van Aken, M. A. C. Stuart and F. Van De Velde, J. Texture Stud., 2007, 38, 511–535 CrossRef.
- G. Sala, T. van Vliet, M. A. Cohen Stuart, G. A. v. Aken and F. van de Velde, Food Hydrocolloids, 2009, 23, 1381–1393 CrossRef CAS.
- D. E. Dunstan, Y. Chen, M. L. Liao, R. Salvatore, D. V. Boger and M. Prica, Food Hydrocolloids, 2001, 15, 475–484 CrossRef CAS.
- M. Srivastava and V. P. Kapoor, Chem. Biodiversity, 2005, 2, 295–317 CAS.
- A. C. Pinheiro, A. I. Bourbon, C. Rocha, C. Ribeiro, J. M. Maia, M. P. Gonçalves, J. A. Teixeira and A. A. Vicente, Carbohydr. Polym., 2011, 83, 392–399 CrossRef CAS.
- M. Mancini, M. Moresi and R. Rancini, J. Texture Stud., 1999, 30, 639–657 CrossRef.
- G. Gentile, F. Greco and D. Larobina, Eur. Polym. J., 2013, 49, 3929–3936 CrossRef CAS.
- Food Texture and Viscosity: Concept and Measurement, ed. M. C. Bourne, Academic Press, California, USA, 2002 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02119c |
| This journal is © The Royal Society of Chemistry 2014 |