Trap states in chemically derived graphene oxide revealed by anomalous temperature-dependent photoluminescence†
Abstract
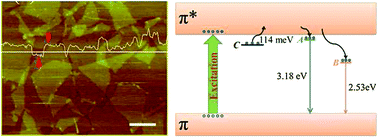
We carry out a comprehensive temperature-dependent photoluminescence (PL) study on chemically derived graphene oxide (GO) sheets. According to the unusual temperature dependence, we introduce a trap state ∼114 meV beneath the LUMO, which implies an additional carrier decay process.

 Please wait while we load your content...
Please wait while we load your content...