Bent-core liquid crystal phases promoted by azo-containing molecules: from monomers to side-chain polymers†
Nélida Gimenoa,
Inmaculada Pintrea,
Marta Martínez-Abadíaa,
José Luis Serranob and
M. Blanca Ros*a
aInstituto de Ciencia de Materiales de Aragón, Universidad de Zaragoza-CSIC, Departamento de Química Orgánica, Facultad de Ciencias, Pedro Cerbuna, 12, 50009 Zaragoza, Spain. E-mail: bros@unizar.es
bInstituto de Nanociencia de Aragón, Universidad de Zaragoza, Departamento de Química Orgánica, Facultad de Ciencias, Pedro Cerbuna 12, 50009 Zaragoza, Spain
First published on 11th April 2014
Abstract
New bent-core monomethacrylates and their side-chain polymers containing the 3,4′-biphenylene moiety as a central core and both all-ester and azo-ester lateral structures have been prepared and characterized. Attractive results concerning the scarcely studied side-chain homo- and copolymers, synthesized either by in situ polymerization in the mesophase or ATRP in solution, are reported. The research is focused on the synthesis of these materials and the effect that structural changes, such as the number of aromatic rings (5 or 6), the presence of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– versus –COO– links on the lateral structures, and the relative positions of the reactive groups, have on the liquid crystal properties of these new bent-core based-materials. The mesomorphic behavior of these low and high-molecular compounds, and also of the synthetic intermediates with bulky terminal bromo-substituents, have been characterized by POM, XRD, DSC and electrooptic studies, showing that the majority of these new molecules have the ability to organize forming non classical lamellar or columnar mesophases. Significant stabilization of the lamellar SmCP mesophase ranges is achieved upon polymerization in comparison to the monomers and interestingly the vitrification of these supramolecular organizations occurs at room temperature.
N– versus –COO– links on the lateral structures, and the relative positions of the reactive groups, have on the liquid crystal properties of these new bent-core based-materials. The mesomorphic behavior of these low and high-molecular compounds, and also of the synthetic intermediates with bulky terminal bromo-substituents, have been characterized by POM, XRD, DSC and electrooptic studies, showing that the majority of these new molecules have the ability to organize forming non classical lamellar or columnar mesophases. Significant stabilization of the lamellar SmCP mesophase ranges is achieved upon polymerization in comparison to the monomers and interestingly the vitrification of these supramolecular organizations occurs at room temperature.
Introduction
Since the first report on bent-core liquid crystals (BCLCs) by Niori et al. in 1996,1 this type of liquid crystalline material has gained increasing interest in many respects, from basic research to innovative applications.2 Despite the possibility that the introduction of this sort of bent-core structure into macromolecular materials would combine the benefits of both systems, most of the research has been focused on low molecular weight materials and examples of macromolecules are rare. The first steps in the route to bent-core-based high molecular weight compounds involves the appropriate design of monomers that contain reactive units capable of either undergoing polymerization reactions or being attached to a preformed polymer or dendrimer. In this aim, reactive groups such as alkenes, 1,3-dienes, acrylates, methacrylates or carboxylic acids have been used. The latter are probably the least common ones, and they are mainly used to incorporate bent-core structures into dendritic macromolecules either by ionic3 or covalent interactions.4In contrast, the alkene units (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) are by far the most common reactive groups to be introduced into bent-core monomers due to their photo/thermal stability and ease of preparation.
C) are by far the most common reactive groups to be introduced into bent-core monomers due to their photo/thermal stability and ease of preparation.
Several series of bent-core monomers bearing one or two alkenyl terminal chains and showing different types of mesophases have been reported5 but only in a few cases their polymerizations have been accomplished. For example, Galli et al.6 used direactive monomers to perform acyclic diene metathesis (ADMET) to provide main-chain polymers containing bent-core units. A different strategy, described by Balamurugan and Kannan et al.,7 involved the epoxidation of the terminal double bonds of the bent-core structures to afford main-chain polyethers. The majority of alkenyl-terminated bent-core molecules have been used to prepare different type of polysiloxane derivatives. For example, monoreactive monomers have provided side-chain homopolymers and copolymers,8 and dendrimers9 by hydrosilylation using Karsted's catalyst or elastomers.10 Likewise, the use of similar reaction conditions have allowed Gimeno et al.11 to obtain bent-core based main-chain polysiloxanes from direactive molecules, by the hydrosilylation polyaddition, to give dark conglomerate organizations. A single example reported by Sentman and Gin12 concerned the thermal and/or radical photopolymerization of 1,3-dienoxy terminal chains to afford liquid crystalline polymeric networks.
Bent-core acrylates and methacrylates have also been used to obtain polymeric systems. These reactive moieties are widely used in other types of liquid crystals but are not common in BCLCs as they may frustrate the mesogenic behaviour when introduced at the end of the terminal chains. Indeed, the early attempts of to functionalize a five-ring bent-core structure with acrylate-terminated chains was reported by Keum et al.13 and gave rise to a non-mesomorphic monomers that were properly mixed with a non-reactive bent-core structure and by photopolymerization, to provide a network that showed a lamellar liquid crystalline organization. However, elongation of the rigid core to a six-ring structure afforded mesogenic monomers (bearing acrylate or methacrylate units) and these were photopolymerized to yield SmCP-like network polymers, as reported by Barberá et al.14 Interestingly, if only one reactive unit (acrylate or methacrylate) is present, mesomorphism can be preserved even for 5 ring monomers, as reported by Li et al.15 These sorts of monomer were polymerized both by free radical polymerization and atom transfer radical polymerization (ATRP) to yield BCLC side-chain polymers that showed a bilayer SmCPA2 phase. Likewise, Barberá et al.16 reported SmCP and Colr mesomorphism for five-ring bent-core polymers obtained by hydrogen bonding.
Herein, we report a new series of monoreactive monomers bearing methacrylate units that can undergo different types of polymerizations (Scheme 1). Interestingly, some of the monomers contain an azo-linking group with the aim of exploiting the well-known photoactive response of this bond and the current interest in azobenzene bent-core molecules.17 To the best of our knowledge, only in the recent work by Kannan et al.17o bent-core reactive monomers bearing azobenzene units have been successfully polymerized in solution. Interestingly, weak AF and F switching and photoisomerization behaviour in thin films have been reported for these low molecular weight polymethacrylates.
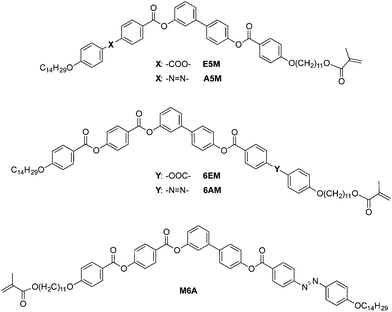
In our case, all of the bent-core compounds reported here are based on a 3,4′-biphenyl central unit and the compounds are named as follows: to differentiate the presence of an azo-benzene unit from the analogous all-ester molecules, the letter A or E is used, respectively; Br is used to denote bromo-derivatives while M represents the methacrylate monomers; in order to define the connecting bonds or terminal groups on the 3,4′-biphenyl system, the compounds are labelled on the left-hand side (for a 3-substituted position) or right-hand side (for a 4′-substituted position) with a number, 5 or 6, which indicates the total number of aromatic rings in the molecule.
For the six-ring systems, the azo moiety is located in the peripheral unit either closest (6AM) or furthest (M6A) from the reactive point, with the aim of assessing the influence of the position of this photosensitive group on the liquid crystalline properties and the photoresponse of the materials. The synthesis and characterization of these new bent-core compounds is reported along with preliminary results on the different polymerization processes, namely photo- and ATR-polymerization.
Results and discussion
Synthesis of monomers
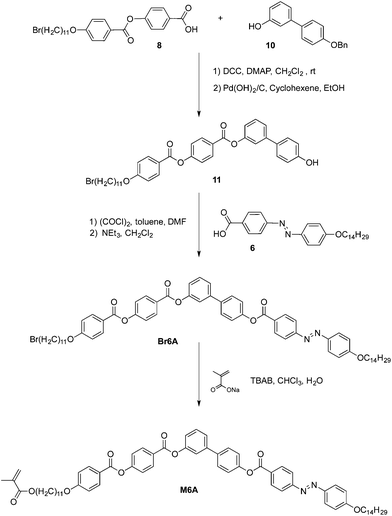
The monomers were prepared by different synthetic pathways depending on the number of aromatic rings and the chemical structures (see Schemes 2–4 and S1†). Five-ring monomers (Scheme 2) were prepared from 3,4′-biphenyl derivatives 3 and 4, which were synthesized and subsequently condensed with ester- or azo-containing benzoic acids 5 and 6 to yield five-ring structures E5Br and A5Br, respectively. These monomers were esterified under phase transfer conditions to give the reactive monomers E5M and A5M. Six-ring structures bearing the azobenzene unit were prepared by two different methods depending on the position of the azo group with respect to the reactive unit (Schemes 3 and 4). Compound 6AM (bearing azo and methacrylate in the same lateral structure) was prepared by esterification of compound 7, with reactive benzoic acid 9 bearing an azo linker. all-Ester monomer 6EM was prepared using a similar strategy to that used to synthesize 6AM, but with the corresponding acid 8 and subsequent incorporation of the reactive group under phase transfer conditions. Monomer M6A (with the azo and methacrylate units in different lateral structures) was prepared by esterification of compound 11, which contains a bromo-substituent at the end of the terminal chain, with non-reactive azo-containing benzoic acid 6 to yield compound Br6A. This was followed by reaction of the bromo-substituent to give the methacrylate under phase transfer conditions. It should be noted that compound Br6A was obtained as a mixture of bromo/chloro derivatives, as shown by MS. The preparation of intermediates 1, 7 and 10 and benzoic acids 5 and 6 has been reported previously.17g,h,18 Details for the synthesis of the new compounds can be found in the ESI.†Liquid crystal characterization
Some of the bent-core intermediates and all of the target reactive monomers were investigated by polarized light optical microscopy (POM) on a hot stage, differential scanning calorimetry (DSC) and variable temperature X-ray diffraction (XRD). The thermal properties and diffraction data for all compounds are gathered in Tables 1 and 2, respectively.| Compound | Phase transition temperature [°C] and enthalpya,b [kJ mol−1] |
|---|---|
| a Data determined by DSC, from second scans at a scanning rate of 10 °C min−1.b Cr, crystal; SmCPA, antiferroelectric smectic C polar mesophase; Bx: unidentified mesophase; Colr rectangular columnar mesophase; I, isotropic liquid.c Joined enthalpy of the SmCP–Colr–I transition.d Maximum of a broad peak.e Mixture of Br/Cl-terminated bent-core compounds. | |
| E5Br | Cr 83.7 [0.4] SmCPA 91.5 [46.0] I |
| I 86.9 [17.3] SmCPA 57.8 [21.6] Cr | |
| E5M | Cr 79.1 [74.3] I |
| I 79.9 [19.7] SmCPA 53.9 [33.4] Cr | |
| A5Br | Cr 96.2 [65.4] I |
| A5M | Cr 95.1 [80.1] I |
| 6EBr | Cr 94.0 [8.4] SmCPA 132.8 [19.3] Colr–Ic |
| I 133.1 [16.6] Colr 124.8 [1.8] SmCPA 66.3 [12.9] Cr | |
| 6EM | Bx 52.7 [4.5] SmCPA 131.4 [22.2] I |
| I 130.2 [22.3] SmCPA 49.0 [3.0] Bx | |
| Br6Ae | Cr 111.5 [52.7] SmCPA 138.7c [14.9] I |
| I 135.0d [14.3] SmCPA 103.4 [60.5] Cr | |
| M6A | Cr 105.7 [49.1] SmCPA 134.4b [16.3] I |
| I 130.5d [17.3] SmCPA 98.5 [59.3] Cr | |
| 6AM | Cr 108.8 [60.3] Colr 138.6 [19.5] I |
| I 137.8 [18.9] Colr 100.2d [64.8] Cr | |
| Compound | Mesophase | dobs (Å) | Miller indices (hkl) | Lattice parameters (Å) |
|---|---|---|---|---|
| E5Br | SmCP | 37.0 | 001 | c: 36.4 |
| 11.9 | 003 | |||
| 6EBr | SmCP | 40.2 | 001 | c: 40.2 |
| Colr | 40.5 | 002 | a: 47 | |
| 39.9 | 101 | b: 79.8 | ||
| 6EM | SmCP | 41.1 | 001 | c: 41.2 |
| 13.8 | 003 | |||
| Bx | 41.1 | 001 | c: 41.2 | |
| 20.6 | 002 | |||
| 13.8 | 003 | |||
| Br6A | SmCP | 41.1 | 001 | c: 41.3 |
| 13.8 | 003 | |||
| M6A | SmCP | 41.1 | 001 | c: 41.4 |
| 13.9 | 003 | |||
| 6AM | Colr | 41.1 | 002 | a: 35.6 |
| 32.7 | 101 | b: 82.2 | ||
| 17.8 | 200 |
Liquid crystalline properties of halogenated bent-core intermediates
It can be seen from Schemes 2–4 that the synthetic routes designed to obtain the desired bent-core monomers involved the preparation of intermediate bent-core structures with terminal bromo-substituents. Three out of the four halogenated bent-core intermediates were liquid crystalline. The shortest compound, which contains an azo linkage, A5Br, was not mesomorphous, whereas the analogous compound with all ester linkages (E5Br) showed an enantiotropic SmCP mesophase, as assigned by the textures observed by POM, electrooptical studies and XRD measurements (see Fig. 1 and Table 2). On cooling from the isotropic liquid, a typical schlieren texture for a SmCPA phase was observed. Moreover, two peaks were observed in the polarization current curve on applying an electric field (triangular voltage 60 V μm−1, 50 Hz) to a sample in a 5 μm Linkam cell and this behaviour indicates antiferroelectric switching (Fig. 1a). Interestingly, on cooling the sample from the isotropic liquid under a strong electric field, regions of different chirality could be detected with slightly uncrossed polarizers after removing the electric field, an observation that is consistent with a dark conglomerate (DC) mesophase induced by the field (Fig. 1b). This phenomenon was also observed for other materials reported here and will be discussed in detail below (see comments on compound Br6A). The lamellar nature of the mesophases was further confirmed by XRD experiments. Two reflections at periodic distances were found in the low angle region and these correspond to a layer spacing of 36.4 Å. This value is far smaller than the theoretical value calculated using Chemsketch® for an all-trans extended conformation of the molecule, 62.2 Å, and supports a tilt of the molecules within the layer.Liquid crystalline behaviour was observed for the six-ring halogenated bent-core structures 6EBr and Br6A. As mentioned previously, Br6A could not be completely purified from a mixture of bromo- and chloro-derivatives. This mixture showed mesogenic behaviour and the phase was assigned as SmCPA on the basis of its electrooptical behaviour. On cooling from the isotropic liquid under an electric field, a texture of focal conic domains appeared and two peaks in the polarization current curve were observed, which indicates antiferroelectric behaviour. Moreover, a higher birefringence was observed along with tilted extinction crosses on applying an electric field, thus confirming an anticlinic arrangement. XRD experiments confirmed the smectic C nature of the mesophase and a layer spacing of 41.3 Å was calculated.
Compound 6EBr showed a broader mesophase range than the azo-material and had very interesting polymorphic behaviour. A texture of circular domains typical of a columnar phase was observed on cooling the sample from the isotropic liquid between two untreated glass substrates. On further cooling, stripes appeared and these correspond to the transition to a lamellar phase (Fig. 2a and b). A 5 μm Linkam cell filled with this material showed different textures on similar treatment. In this case, the columnar phase appeared as a green banana leaves texture that changed to a yellowish schlieren texture on reaching the SmCP phase (see Fig. 2c and d). A detailed study was performed on the columnar and lamellar phases of the compound under an electric field and by X-ray diffraction. The electrooptical observations allowed us to assign the lamellar phase as SmCAPA. It was also possible to induce an irreversible Col–SmCP transition by applying an electric field in the columnar phase. Interestingly, for this material it was feasible to induce either racemic organization or a homochiral dark conglomerate (DC) texture depending on the electric field applied. A homochiral DC phase could be achieved when the sample was cooled down from the isotropic liquid on applying a strong electric field (80–100 V). Under these conditions, the columnar phase is suppressed and only the SmCP phase was formed, as evidenced by a fan-shaped texture. On removing the electric field the texture became dark and this is typical of the homochiral-DC (see Fig. 2e). The same type of homochiral texture is observed when the SmCP is field-induced from the Col mesophase and then the field is removed. Finally, when the field was applied once the SmCP had been reached, only a racemic DC phase was observed on removing the field.
XRD studies allowed us to confirm the columnar and lamellar character of the phases observed. For the high temperature phase, two sharp reflections in the low angle region were observed along with a diffuse halo. The low angle reflection could be indexed to a rectangular cell with parameters a: 47 Å and c: 79.8 Å, thus confirming a columnar arrangement. However, on further cooling, only one sharp reflection in the low angle region was observed and this is consistent with the characteristic lamellar arrangement of a SmCP phase. Moreover, a layer spacing of 40.2 Å was measured. This value is markedly smaller than the theoretical molecular length in an elongated all-trans conformation calculated using Chemsketch. This is consistent with a SmCP mesophase in which the molecules are tilted by 53° within the layers.
Liquid crystalline properties of reactive bent-core monomers
All of the monomers formed bent-core liquid crystalline phases with the exception of the shortest example with the azo unit (A5M), in accordance with the properties observed for the halogenated analogues. On the other hand, the presence of a methacrylate group in E5M led to the formation of liquid crystalline order only on cooling from the isotropic phase. Thus, in contrast to E5Br the monotropic SmCP mesophase could only be observed over a few degrees and it was identified by the schlieren texture observed by POM. Unfortunately, XRD data could not be obtained due to crystallization of the sample.However, enlargement of the rigid bent-core part of the molecule (six aromatic rings) led to the stabilization of the mesomorphism and markedly broadened the temperature range of the mesophase, even for the azo-bent-core monomers. A schlieren texture was formed on cooling 6EM from the isotropic liquid and this is typical of the SmCP phase. Moreover, XRD studies on the mesophase confirmed the lamellar character of the phase as several reflections were observed at periodic distances in the low-angle region along with a diffuse halo in the wide-angle region (Fig. 4a). This compound also had a low temperature phase with a similar lamellar XRD diffraction pattern, but in this case two sharp reflections appeared the wide-angle region, indicating a higher order phase. However, this phase is denoted as Bx as further studies are needed to identify this phase properly.
Azo-based monomers (6AM and M6A) form columnar or lamellar phases depending on the position of the azo moiety with respect to the reactive group (Fig. 3). Thus, compound M6A, in which the azo-group is further from the reactive point, exhibited a schlieren texture on cooling the isotropic liquid and this is consistent with a SmCP mesophase. In addition, the XRD patterns showed two reflections at periodic distances, again in accordance with a lamellar mesophase (Fig. 3a). However, compound 6AM, in which the azo-bond is closer to the methacrylate group, on cooling from the isotropic liquid showed circular domains and a fan-shaped texture, which is characteristic of some bent-core columnar mesophases. The nature of this phase was also confirmed by XRD diffraction. The patterns showed two reflections in the low-angle region that could be indexed to a rectangular cell with parameters a: 35.6 Å and b: 82.2 Å (Fig. 4b). Unfortunately, further studies on the electrooptical behaviour of the reactive materials could not be performed as these reactive molecules polymerized thermally on filling the cells by capillarity in the liquid phase due to their high melting points, even when a thermal inhibitor was used.
 | ||
| Fig. 3 Textures of (a) compound M6A at 100 °C in the SmCP; (b) compound 6EM at 124 °C in the SmCP and (c) compound 6AM at 136 °C in the Colr. | ||
 | ||
| Fig. 4 X-ray diffractograms of (a) 6EM in the SmCP phase, (b) low angle region of 6AM in the Colr phase. | ||
Polymers
Methacrylates are able to undergo different types of polymerization reaction to give side-chain polymers. Among them, photopolymerization or ATRP are suitable alternatives. In particular, in situ photopolymerization, which has been widely used in the liquid crystals field, allows ordered and even aligned thin films of macromolecules to be obtained as the polymerization can take place in the mesophase.14,16,19 On the other hand, ATRP in solution allows more control of the polymer size and gives a low polydispersity. With this in mind, preliminary studies were carried out on the polymerization of some of the bent-core monomers reported here with the aim of obtaining bent-core based side-chain polymethacrylates (Fig. 5). Monomers 6EM, 6AM and M6A were employed in in situ photopolymerizations in the mesophase in order to obtain homopolymers. Additionally, and as a way to modulate optical properties and applications of azo-polymers on decreasing the azo-content, we also applied this technique to obtain random copolymers from these azo-monomers (M6A or 6AM) and the all-ester monomer 6EM. Finally, as the five-ring monomers A5M and E5M do not form stable mesophases, they were not suitable for in situ polymerization in the liquid crystal phase. As a consequence, ATRP was assessed with these monomers and a copolymer from A5M and E5M was prepared.Thermal and XRD data obtained for homopolymers and copolymers are gathered in Table 3. All of the new macromolecules form liquid crystalline phases over a wide temperature range. The results indicate that the mesomorphism is stabilized after polymerization by more than 60 °C. This finding is in contrast with the low stabilization reported by Kannan et al.17o on going from the monomer to the polymer. Moreover, most of the materials were not crystalline in nature and showed a glass transition at around 45 °C (see Fig. S4†), the exceptions being P-M6A and P-M6A, for which the coexistence of glass mesophase and crystalline order was detected. The textures observed by POM indicate a SmCP mesophase and this was confirmed by XRD experiments (Fig. 6 and S5†). All of the polymers were studied at room temperature after a thermal treatment that involved heating the sample to the isotropic liquid and cooling it down to room temperature. In all cases, a diffuse halo in the wide-angle region and up to 5 reflections at periodic distances in the small-angle region were detected. These results confirmed the lamellar nature of the mesophase of these side-chain polymers.
| Polymer | Phase transition temperature [°C] and enthalpya,b [kJ mol−1] | dobse (Å) | Miller indices (hkl) | Lattice parameters (Å) |
|---|---|---|---|---|
| a DSC data from the third scan at a scanning rate of 20 °C min−1.b Cr, crystal; SmCPg glassy polar smectic C phase; SmCP, polar smectic C mesophase; I, isotropic liquid.c Maximum of a peak.d Semicrystalline polymer only detected in DSC studies.e Data measured at room temperature, after a thermal treatment that consisted on heating up the polymer to the isotropic liquid and cooling down to room temperature.f The unique polymer prepared by ATRP in solution. | ||||
| P-6EM | SmCPg 47.7 SmCP 210.0c [17.5] I | 86.0 | 001 | c: 85.7 |
| 42.8 | 002 | |||
| 28.7 | 003 | |||
| 17.2 | 005 | |||
| 14.2 | 006 | |||
| P-M6A | Cr 116.4b,c,d [1.9] SmCP 214.9c [12.6] I | 92.2 | 001 | c: 92.1 |
| 45.6 | 002 | |||
| 30.8 | 003 | |||
| 18.4 | 005 | |||
| 15.5 | 006 | |||
| P-6AM | Cr 97.3b,c,d [4.7] SmCP 221.6c [16.8] I | 89.0 | 001 | c: 89.3 |
| 44.3 | 002 | |||
| 30.1 | 003 | |||
| 17.9 | 005 | |||
| 15.0 | 006 | |||
| P-(6EM+M6A) | SmCPg 46.3 SmCP 211.3c [19.3] I | 85.8 | 001 | c: 85.9 |
| 42.7 | 002 | |||
| 28.7 | 003 | |||
| 17.2 | 005 | |||
| 14.4 | 006 | |||
| P-(6EM+6AM) | SmCPg 46.5 SmCP 211.6c [19.2] I | 82.1 | 001 | c: 82.2 |
| 41.1 | 002 | |||
| 27.3 | 003 | |||
| 16.2 | 005 | |||
| 14.0 | 006 | |||
| P-(E5M+A5M)f | SmCPg 78.8 SmCP 150.7c [7.3] I | 41.1 | 001 | c: 81.7 |
| 27.3 | 002 | |||
| 20.5 | 003 | |||
| 16.2 | 005 | |||
| 13.6 | 006 | |||
 | ||
| Fig. 6 Textures of side-chain polymers (a) P-M6A in the SmCP mesophase at 150 °C; and (b) P-(6EM+6AM) at 211 °C at the isotropic liquid-SmCP mesophase transition. | ||
It is worth mentioning that a SmCP mesophase was observed for P-6AM even though the monomer showed a Colr mesophase. Likewise, the layer spacing measured for these polymers (around 80–90 Å) is double those observed for the monomers and are significantly higher than the theoretical molecular length of the single monomers (around 60 Å). These values can be explained by the formation of bilayers upon polymerization and they are consistent with previous results reported for other bent-core liquid crystalline polymers.14–16
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 with a polydispersity of 1.13 (Mw/Mn) was calculated by SEC for the new copolymer P-(A5M+E5M). Unfortunately, the molecular weight of the material could not be confirmed by 1H-NMR spectroscopy due to the complexity of the spectrum. Regarding the thermal behaviour, the copolymer P-(A5M+E5M) exhibited an enantiotropic mesophase over a wide temperature range (see Table 3), a situation in contrast to the monomers. The mesophase was assigned by XRD as the textures observed by POM were not clear. The X-ray diffractogram showed a diffuse halo in the wide-angle region and up to five reflections at periodic distances in the low-angle region. This pattern is consistent with a lamellar arrangement of the molecules and the mesophase was assigned as SmCP (Fig. S5†). Moreover, very similar diffractograms were obtained at room temperature and in the mesophase, thus confirming the vitrification of the SmCP detected by DSC. Regarding the layer spacing, the calculated experimental value indicates a bilayer structure similar to that proposed for the polymers obtained by in situ polymerization.
700 with a polydispersity of 1.13 (Mw/Mn) was calculated by SEC for the new copolymer P-(A5M+E5M). Unfortunately, the molecular weight of the material could not be confirmed by 1H-NMR spectroscopy due to the complexity of the spectrum. Regarding the thermal behaviour, the copolymer P-(A5M+E5M) exhibited an enantiotropic mesophase over a wide temperature range (see Table 3), a situation in contrast to the monomers. The mesophase was assigned by XRD as the textures observed by POM were not clear. The X-ray diffractogram showed a diffuse halo in the wide-angle region and up to five reflections at periodic distances in the low-angle region. This pattern is consistent with a lamellar arrangement of the molecules and the mesophase was assigned as SmCP (Fig. S5†). Moreover, very similar diffractograms were obtained at room temperature and in the mesophase, thus confirming the vitrification of the SmCP detected by DSC. Regarding the layer spacing, the calculated experimental value indicates a bilayer structure similar to that proposed for the polymers obtained by in situ polymerization.Conclusions
In summary, nine new bent-core 3,4′-biphenylene-based compounds have been prepared by different synthetic strategies. Intermediates with terminal bromo-substituents have been used to prepare five new monomethacrylate molecules, which are characterized by the presence of 5 or 6 aromatic rings and all-ester or azo/ester-linked lateral structures. Interestingly, the majority of these compounds form SmCPA or Colr mesophases, as determined by POM, DSC, XRD and electrooptics studies. The reactive nature and possibilities for these monomethacrylated bent-core molecules have been successfully demonstrated with the aim of providing bent-core side-chain homo- or random copolymers. For this purpose, two alternative polymerization routes have been used, namely in situ photopolymerization in the bent-core mesophase (SmCP or Colr) of pure monomers or blends, or ATRP in solutions. Both of these approaches gave a series of side-chain polymethacrylates that show either glassy SmCP mesophases at room temperature or SmCP mesophases over very broad temperature ranges. These mesophases are stable up to 150 °C in the case of the five-ring bent-core polymers or up to 210 °C for the six-ring systems. These results represent the first steps to explore the potential of this scarcely studied type of bent-core macromolecule. Of particular interest are the wide possibilities of photoresponsive azo-bent-core side-chain polymers in applications such as photoalignment, light-induced chirality or photorecording, which can be studied for glassy and liquid crystalline macromolecules.20Acknowledgements
This work was financially supported by MICINN-FEDER and MINECO-FEDER of Spain-UE (Projects MAT2009-14636-C03-01, MAT2011-27978-C020-1 and MAT2012-38538-C03-01) and the Aragón Government (Project E04).The authors acknowledge JAEDoc-CSIC (N. Gimeno) and JAEPredoc-CSIC (M. Martínez-Abadia) programs. Thanks are given to Nuclear Magnetic Resonance, Mass Spectra and Thermal Analysis Services from the Instituto de Ciencia de Materiales de Aragón, Universidad de Zaragoza – CSIC (Spain). We are grateful to Prof. César Folcia of the Universidad del País Vasco for his assistance in some XRD studies.References
- T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, J. Mater. Chem., 1996, 6, 1231 RSC.
- (a) W. Wesissflog, H. N. Sheenivasa Murthy, S. Diele and G. Pelzl, Philos. Trans. R. Soc., A, 2006, 364, 2657 CrossRef PubMed; (b) R. Amaranatha Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907 RSC; (c) G. Pelzl and W. Weissflog, Thermotropic Liquid Crystals. Recent Advances, ed. A. Ramamoorthy, Springer, The Netherlands, 2007, ch. 1, p. 1 Search PubMed; (d) A. Jakli, C. Bailey and J. Harden, Thermotropic Liquid Crystals. Recent Advances, ed. A. Ramamoorthy, Springer, The Netherlands, 2007, ch. 2, p. 59 Search PubMed; (e) J. Etxebarria and M. B. Ros, J. Mater. Chem., 2008, 18, 2919 RSC; (f) S. K. Lee, X. D. Li, S. M. Kang, M. Tokita and J. Watanabe, J. Mater. Chem., 2009, 19, 4517 RSC; (g) A. Eremin and A. Jakli, Soft Matter, 2013, 9, 615 RSC.
- J. Vergara, N. Gimeno, M. Cano, J. Barberá, P. Romero, J. L. Serrano and M. B. Ros, Chem. Mater., 2011, 23, 4931 CrossRef CAS.
- (a) D. Kardas, M. Prehm, U. Baumeister, D. Pociecha, R. Amaranatha Reddy, G. H. Mehl and C. Tschierske, J. Mater. Chem., 2005, 15, 1722 RSC; (b) N. Gimeno, J. Vergara, M. Cano, J. L. Serrano, M. B. Ros, J. Ortega, C. L. Folcia S. Rodríguez-Conde, G. Sanz-Enguita and J. Etxebarria, Chem. Mater., 2013, 25, 286 CrossRef CAS.
- (a) K. Fodor-Csorba, A. Vajda, G. Galli, A. Jákli, D. Demus, S. Holly and E. Gács-Baitz, Macromol. Chem. Phys., 2002, 203, 1556 CrossRef CAS; (b) K. Fodor-Csorba, A. Vajda, A. Jákli, C. Slugov, G. Trimmel, D. Demus, E. Gács-Baitz, S. Holly and G. Galli, J. Mater. Chem., 2004, 14, 2499 RSC; (c) V. Novotná, V. Hamplová, M. Kašpar, M. Glogarová and D. Pociecha, Liq. Cryst., 2005, 32, 1115 CrossRef; (d) R. Achten, E. A. W. Smits, R. A. Reddy, M. Giesbers, A. T. M. Marcelis and E. J. R. Sudhölter, Liq. Cryst., 2006, 33, 57 CrossRef CAS; (e) V. Kozmík, A. Kovářová, M. Kuchař, J. Svoboda, V. Novotná, M. Glogarová and J. Kroupa, Liq. Cryst., 2006, 33, 41 CrossRef; (f) L. Rahman, J. Asik, S. Kumar and C. Tschierske, Liq. Cryst., 2008, 35, 1263 CrossRef CAS.
- G. Galli, S. Demel, C. Slugovc, F. Stelzer, W. Weissflog, S. Diele and K. Fodor-Csorba, Mol. Cryst. Liq. Cryst. Cryst., 2005, 439, 1909 CAS.
- S. Balamurugan and P. Kannan, J. Mol. Struct., 2009, 934, 44 CrossRef CAS PubMed.
- (a) C. Keith, R. A. Reddy and C. Tschierske, Chem. Commun., 2005, 871 RSC; (b) R. Achten, A. Koudijs, M. Giesbers, R. Amaranatha Reddy, T. Verhulst, C. Tschierske, A. Marcelis and E. Sudhölter, Liq. Cryst., 2006, 33, 681 CrossRef CAS; (c) A. Bubnov, V. Novotná, D. Pociecha, M. Kaåpar, V. Hamplová, G. Galli and M. Glogarová, Macromol. Chem. Phys., 2011, 212, 191 CrossRef CAS.
- (a) H. Hahn, C. Keith, H. Lang, R. A. Reddy and C. Tschierske, Adv. Mater., 2006, 18, 2629 CrossRef CAS; (b) C. Keith, R. A. Reddy, M. Prehm, U. Baumeister, H. Kresse, J. L. Chao, H. Hahn, H. Lang and C. Tschierske, Chem. –Eur. J., 2007, 13, 2556 CrossRef CAS PubMed; (c) C. Keith, G. Dantlgraber, R. A. Reddy, U. Baumeister, M. Prehm, H. Hahn, H. Lang and C. Tschierske, J. Mater. Chem., 2007, 17, 3796 RSC.
- R. Verduzco, P. Luchette, S. H. Hong, J. Harden, E. DiMasi, P. Palffy Muhoray, S. M. Kilbey II, S. Sprunt, J. T. Gleeson and A. Jákli, J. Mater. Chem., 2010, 20, 8488 RSC.
- N. Gimeno, A. Sánchez-Ferrer, N. Sebastián, R. Mezzenga and M. B. Ros, Macromolecules, 2011, 44, 9586 CrossRef CAS.
- A. C. Sentman and D. L. Gin, Angew. Chem., Int. Ed., 2003, 42, 1815 CrossRef CAS PubMed.
- C. D. Keum, A. Kanazawa and T. Ikeda, Adv. Mater., 2001, 13, 321 CrossRef CAS.
- (a) J. Barberá, N. Gimeno, L. Monreal, R. Piñol, M. B. Ros and J. L. Serrano, J. Am. Chem. Soc., 2004, 126, 7190 CrossRef PubMed; (b) B. Atorf, A. Hoischen, M. B. Ros, N. Gimeno, C. Tschierke, G. Dantlgraber and H. Kitzerow, Appl. Phys. Lett., 2012, 100, 223301 CrossRef PubMed.
- X. Chen, K. K. Tenneti, C. Y. Li, Y. Bai, X. Wan, X. Fan, Q. F. Zhou, L. Rong and B. S. Hsiao, Macromolecules, 2007, 40, 840 CrossRef CAS.
- J. Barberá, N. Gimeno, I. Pintre, M. B. Ros and J. L. Serrano, Chem. Commun., 2006, 1212 RSC.
- (a) V. Prasad, Liq. Cryst., 2001, 28, 145 CrossRef CAS; (b) V. Prasad, D. S. S. Rao and S. K. Prasad, Liq. Cryst., 2001, 28, 643 CrossRef CAS; (c) V. Prasad, Mol. Cryst. Liq. Cryst., 2001, 363, 167 CrossRef CAS; (d) V. Prasad and A. Jákli, Liq. Cryst., 2004, 31, 473 CrossRef CAS; (e) V. Prasad, S. W. Kang, X. Qi and S. J. Kumar, J. Mater. Chem., 2004, 14, 1495 RSC; (f) V. Prasad, S. W. Kang, K. A. Suresh, L. Joshi, Q. Wang and S. Kumar, J. Am. Chem. Soc., 2005, 127, 17224 CrossRef CAS PubMed; (g) C. L. Folcia, I. Alonso, J. Ortega, J. Etxebarria, I. Pintre and M. B. Ros, Chem. Mater., 2006, 18, 4617 CrossRef CAS; (h) I. C. Pintre, N. Gimeno, J. L. Serrano, M. B. Ros, I. Alonso, C. L. Folcia, J. Ortega and J. Etxebarria, J. Mater. Chem., 2007, 17, 2219 RSC; (i) M. R. Lutfor, G. Hegde, S. Kumar, C. Tschierske and V. G. Chigrinov, Opt. Mater., 2009, 32, 176 CrossRef CAS PubMed; (j) L. Rahman, S. Kumar, C. Tschierske, G. Israel, D. Ster and G. Hegde, Liq. Cryst., 2009, 36, 397 CrossRef CAS; (k) M. Vijay Srinivasan, P. Kannan and A. Roy, Liq. Cryst., 2012, 39, 1465 CrossRef CAS; (l) N. G. Nagaveni, A. Roy and V. Prasad, J. Mater. Chem., 2012, 22, 8948 RSC; (m) M. Alaasar, M. Prehm and C. Tschierske, Liq. Cryst., 2013, 40, 656 CrossRef CAS; (n) M. Alaasar, M. Prehm, M. Nagaraj, J. K. Vij and C. Tschierske, Adv. Mater., 2013, 25, 2186 CrossRef CAS PubMed; (o) M. Vijay Srinivasan, P. Kannan and A. Roy, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 936 CrossRef; (p) M. Horčic, V. Kozmík, J. Svoboda, V. Novotná and D. Pociecha, J. Mater. Chem. C, 2013, 1, 7560 RSC; (q) M. Alasaar, M. Prehm and C. Tschierske, Liq. Cryst., 2014, 41, 126 CrossRef.
- G. Dantlgraber, A. Eremin, S. Diele, A. Hauser, H. Kresse, G. Pelzl and C. Tschierske, Angew. Chem., Int. Ed., 2002, 41, 2408 CrossRef CAS.
- (a) R. A. M. Hikmet, Adv. Mater., 1992, 4, 679 CrossRef CAS; (b) D. J. Broer, J. Lub and G. N. Mol, The Wiley Polymer Network Group Review Series, ed. K. T. Nijenhuis and W. J. Mijs, Wiley, New York, 1998, vol. 1, p. 361 Search PubMed; (c) I. Dierking, Adv. Mater., 2000, 12, 167 CrossRef CAS; (d) C. Artal, M. B. Ros, J. L. Serrano, M. R. de la Fuente and M. A. Pérez Jubindo, Chem. Mater., 2001, 13, 2056 CrossRef; (e) C. Artal, M. B. Ros, J. L. Serrano, N. Pereda, J. Etxebarria, C. L. Folcia and J. Ortega, Macromolecules, 2001, 34, 4244 CrossRef CAS; (f) H. R. Stapert, H. S. del Valle, E. J. K. Verstegen, B. M. I. Van der Zande, J. Lub and S. Stallinga, Adv. Funct. Mater., 2003, 13, 732 CrossRef CAS.
- (a) A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139 CrossRef CAS PubMed; (b) A. S. Matharu, S. Jeeva and P. S. Ramanujam, Chem. Soc. Rev., 2007, 36, 1868 RSC; (c) T. Ikeda, J. Mamiya and Y. L. Yu, Angew. Chem., Int. Ed., 2007, 46, 506 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and techniques. MS of compound Br6A. Thermal properties of the photopolymerizable mixtures. 1H-NMR studies of polymer P-M6A. DSC thermograms and POM images of representative monomers and polymers. See DOI: 10.1039/c4ra02079k |
| This journal is © The Royal Society of Chemistry 2014 |