Sketching functional, ubiquitous ZnO nano-sensors on paper
N. Mohseni Kiasari*,
S. Soltanian,
B. Gholamkhass and
P. Servati
Flexible Electronics and Energy Lab (FEEL), Department of Electrical and Computer Engineering, University of British Columbia, 2332 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada. E-mail: nimamk@ece.ubc.ca
First published on 15th April 2014
Abstract
We report the fabrication of functional nanoelectronic sensors on regular copy paper by simply sketching electrode lines and painting the resistively sensing nanostructured film using synthesized zinc oxide (ZnO) nanoparticle (NP) inks. A repeatable three orders of magnitude increase in conductance is demonstrated when the sensors are exposed to 30 s long 1220 μW cm−2 ultra-violet (UV) irradiation pulses in the dark with a 6 s and 3 s rise and recovery times, respectively. Moreover, a 200% increase in current is also observed at room temperature, when these sensors are exposed to successive pulses of pure CO. The sensors exhibit a fast response to variations in concentration of CO as small as a 1% volume change. The sensitivity to CO concentration can be significantly improved by using UV irradiation. This work shows that sketching of metal oxide nanostructures with controlled nanoscale properties on paper can be employed for fabrication of accurate and reliable sensors that can find applications in ubiquitous environmental sensing, electronic paper, wearable electronics, and sensor networks.
1. Introduction
The increasing role of mobile electronic devices in our daily life has highlighted the demand for ubiquitous sensors that enable accurate sensing of different stimuli in the environment.1 From CO monitoring of home furnaces and fireplaces to improved security, ubiquitous sensors for environmental monitoring have significant potential market and impact for our daily life. The cost and complexity of material systems, substrates and fabrication process of ubiquitous sensors must be prohibitively low, without compromising the functionality and accuracy of the sensors. Among different substrates, paper is used in many shapes or forms (e.g., print paper, paper bags, packaging, and cups) in our daily life,2 costs less than one tenth of the cost of any plastic substrates,3 is recyclable and environment-friendly, and adaptable with a variety of conventional and novel printing and painting techniques.4,5 Due to its fibrous composition, it provides a high surface area that is suitable for many applications, particularly sensing and filtration. Moreover, paper's cellulose fibrous matrix shows a strong bonding to a broad range of nano-ink materials.6,7Metal oxide nanostructures, including zinc oxide (ZnO), are well-known for their sensing properties due to the presence of surficial oxygen vacancies,8,9 high surface-to-volume ratios,10 morphological diversity (e.g., nanowires, nanorods, and nanoparticles) and variety of synthesis methods (e.g., such as chemical vapour deposition (CVD), laser ablation, hydrothermal, and sol–gel process). While precursor materials for ZnO nanostructures have low cost, the main obstacle in materialization of commercial ZnO nanoelectronic sensors is the complexity of fabrication methods used for development of functional and accurate devices. Most methods involve sophisticated and expensive patterning techniques such as electron beam lithography that has fairly low yields and smooth and clean substrates. Despite the potential for fabrication of low cost ZnO based sensors on paper substrates, there is limited work in the published literature. Gimenez et al.7 have investigated ZnO particulate films on paper with pencil drawn electrodes. Gullapalli et al.11 have demonstrated strain-sensing devices using ZnO nanorods synthesized directly on paper cellulose fibers to form a nanocomposite material. Antibacterial activity of ZnO nanostructures on paper is investigated in another work.6
In this work, we present nanoelectronic sensors sketched on paper from solution synthesized ZnO NP inks. Combining a fine hand brush, a custom stamp and custom-made calligraphy ink, we sketch the electrodes used for fabrication of our sensor devices. The sketched ZnO sensors demonstrate significant sensitivity to UV exposure and highly repeatable, fast transient response to UV due to the nanoscale features of ZnO nanoparticles and uniformity of film and good bonding to paper and electrodes. The devices show a high sensitivity to CO concentration (as small as 1% change in the concentration in air) in the ambient at room temperature and improved sensitivity to CO under UV irradiation. As a result, this work demonstrates the potential for simple sketching techniques using synthesized ZnO and electrode inks, in comparison to complex and costly fabrication methods, for integration of sensitive devices with repeatable function on paper for UV and CO sensing. We believe this work along with other reports pave the way toward low-cost fabrication of functional, ubiquitous electronic and sensing devices, which can be extended to broad range of substrates and materials.11
2. Device fabrication
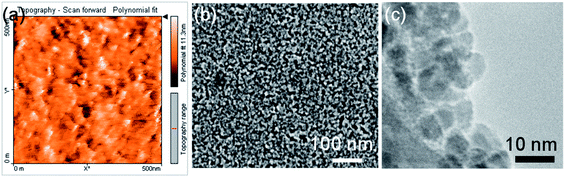
ZnO nanoparticles were synthesized following the work of Pacholski et al.12 First, zinc acetate dihydrate (Sigma-Aldrich, 2.95 g) was dissolved in methanol (125 mL) on a stirring hot plate at 120 °C (actual solution temperature was ∼60 °C) with stirring speed of 200 rpm. A solution of potassium hydroxide (Fischer Scientific, 1.48 g) in methanol (65 mL) was slowly added over 10 min. Zinc hydroxides precipitated but dissolved again. After almost 2 hours, the NPs started to precipitate and both heating and stirring were stopped. The NPs were washed with methanol 2 times and in the final stage were dissolved in 10 mL of chloroform yielding an almost transparent suspension of NPs, which remains stable for two weeks, with an approximate concentration of 90 mg mL−1, estimated by drying a sample solution and weighing the resulting ZnO. The microscopic structure of the NPs was characterized by a Nanosurf EasyScan Atomic Force Microscope (AFM) and a Zeiss Sigma Field Emission Scanning Electron Microscope (FESEM). As shown in Fig. 1a and b, the roughness of a spin coated layer of NPs is about 10 nm and NPs are uniformity distributed on the surface. Finally, the NP morphology was studied by FEI Tecnai Transmission Electron Microscope (TEM) (Fig. 1c) confirming the uniformity of NPs with an average size of ∼7–8 nm.Since the resistance of a typical sketched device (as shown in Fig. 2a) is expected to be a few GΩ, an electrode with resistance of up to a few MΩ minimally affects the total electronic performance of the device. This gives us freedom in choosing the electrode from highly conductive evaporated metals to low-cost alternatives such as pencil line and calligraphy ink. Among all the electrode options, custom-made calligraphy ink was preferred for this work due to the low cost, strong adhesion and bonding to paper, and compatibility with printing and sketching techniques. The resulting electrode lines exhibit stronger adhesion to paper in comparison to the lines drawn by pencil. It was observed that the conductivity of the electrode lines sketched using calligraphy ink, unlike pencil lines, does not show any change after NP ink deposition, which contains chloroform, or even by mechanical bending of the substrate. The ink is made based on a traditional recipe by using equal volume ratios of lampblack (Graphic Chemical & Ink Co.) as the black pigments, and gum arabic (Sigma Aldrich), which not only helps the carbon particles stabilize but also makes the ink more viscous and results in adhesion of the carbon particles to the paper. First the gum arabic was dissolved in deionized water to form a thick syrup-like solution then lampblack was added to it. Moreover, lampblack is composed of carbon particles as small as 10 nm in size, fusing into each other during the combustion and can form stable nano-clusters of 100–300 nm in size13 which was also confirmed by SEM micrograph as shown in the Fig. 2d.
3. Results and discussion
The regular copy paper is extremely porous and composed of randomly oriented micrometer-sized cellulose fibers. The rough surface is not suitable for various fabrication processes required for many electronic devices. However, it is observed7 to be a suitable substrate for our application as it provides a porous matrix which holds the NP ink and increases the sensing surface area interacting with the surrounding environment.As displayed in Fig. 2a, sketching steps of a sensor on copy paper are; first using a custom made stamp with six 1 inch long and 1 mm wide electrode lines with a 2 mm pitch. Then three of the lines are connected using the ink and a brush. Although all the lines can be drawn using a brush, we used a stamp to have devices with closely repeatable structure for comparison. Each stamped line has a resistance of 150–250 kΩ. Once the electrode lines are drawn, then ZnO NP ink is painted using a brush. Although precise control over the amount of ZnO deposition is not possible as the sensors are prepared by hand, fairly consistent results are obtained by passing the brush 4–6 times over the device area. The average amount of ZnO NPs deposited on the device area is estimated by weighing the device before and after brushing and obtained to be ∼2.7–3.5 mg cm−2. These devices show a long lifetime as they exhibit to be operational after 1 month, and more importantly, by deflecting or rolling over the surface of a pencil (with a radius of ∼2.5 mm) the sketched devices still function. This mechanical stability is originated from the strong bonding and adhesion of the paper cellulose fibers to the ZnO NP and electrode's graphite NPs. More comprehensive studies on the mechanical stability, flexibility, and life-time of these devices are under investigation.
3.1. Ultra violet sensing
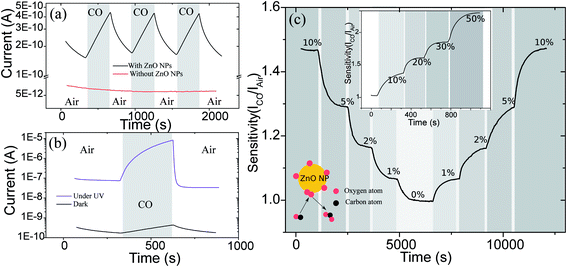
ZnO is strongly absorbs ultra-violet (UV) irradiation due to electronic transition within its wide band gap (3.37 eV (ref. 14)). A typical device made on paper was exposed to a UV lamp with a peak wavelength at 365 nm and power intensity of 1220 μW cm−2 at 4 cm distance, measured by a Newport 818-UV photo-detector. Fig. 3a illustrates the steady state current–voltage characteristics in linear and logarithmic scale (in-set) of a typical device measured using a Keithley 4200 semiconductor analyzer in the dark and under UV irradiation. As seen, the current increases almost 2000 times under UV irradiation. The physics behind this increase in current has been studied extensively15–17 and can be attributed to two different mechanisms. First mechanism is the sequential absorption of the UV photon, a band-to-band transition resulting in electron–hole-pair (EHP) generation (depicted schematically in the inset of Fig. 3a). Since the surface of zinc oxide is covered with negatively charged oxygen species, there is a band bending at the surface of ZnO. When exposed to UV radiation, as a result of the increased density, holes diffuse toward the surface of NPs in the proximity of this band bending and release some of the previously adsorbed oxygen at the surface of NPs.18 These two mechanisms result in a significant boost in conductivity of the ZnO film, which is in agreement with other works on ZnO UV sensing.16,17In order to study the transient behavior of these devices to UV irradiation, a 30 s long UV pulse was irradiated to a sensor and the current was measured as shown in Fig. 3b. The response of the sensor demonstrates is fast with a rise (10% to 90% of final value) and fall times (90% to 10%) of 6 s and 3 s, respectively. The fast response of our sensors compared to other nano-ZnO UV detectors17,19–21 can be attributed to the small size of the NPs used in the sensors. The effect of the intensity of UV light on the sensor was also examined. For this purpose UV light with different intensities was applied to the devices and the transient current of the device was measured accordingly. As illustrated in Fig. 3c, initially the highest intensity, 1220 μW cm−2, was applied and then decreased step by step to a minimum at 220 μW cm−2, and again increased step by step to the initial value. As shown, the sensor relative response, current normalized to the current measured for the minimum light intensity, follows the changes in the light intensity (the inset of Fig. 3c) and the current stays stable at each intensity value. The sensor exhibits the same signal when the intensity returns back to its initial value.
3.2. Carbon monoxide sensing
Zinc oxide like other metal oxides is sensitive to carbon monoxide (CO) in the environment, mainly due to a reaction with the negatively charged oxygen species at the surface of the metal oxide, in this case ZnO, which results in the release of electrons, and increase the carrier density of the ZnO.22 For ZnO, the reaction rate is very low at room temperature.22,23 Therefore, obtaining a measurable response usually requires either high temperature or alternative methods, such as surface doping of the ZnO, to facilitate the reaction process.22,24,25 Unlike the results reported in the literature so far, it was observed that our sensors show a fast and strong response to CO environment at room temperature as illustrated in Fig. 4. This interesting behaviour can be attributed to the small size of NPs (∼7–8 nm) and the fact that a large proportion of atoms are surface atoms and therefore any reaction at the surface can considerably influence the electronic behaviour of the whole NP.As displayed in the Fig. 4a, a 5 min exposure to CO environment results in 200% increase in the current (ICO/Iair = 3) of the device. A control device was also fabricated by drawing the electrode lines on a paper substrate without the ZnO layer and as shown in the Fig. 4a (red line) there is no change in the conductivity of the paper in the presence of CO. This confirms that the sensitive behaviour is due to the ZnO NP layer and rules out the role of the paper substrate or the electrode line in the observed sensitivity to CO. The slow rise and decrease in current can be attributed to the slow rate of the reaction of the oxygen species and CO in the environment. Interestingly, it was observed that exposure to UV irradiation increases the sensitivity to CO (S = ICO/Iair), as well as, the response and recovery times, which improves from 180 to 5 s for the latter, as shown in the Fig. 4b. Under UV irradiation with an approximate intensity of 250 μW cm−2, the sensitivity of the device increases more than 37 times (after 5 minutes exposure to CO), when compared to a device tested for similar CO exposure in the dark. This behavior can be attributed to the formation of more reactive photo-induced oxygen ions at the surface of the ZnO26,27 that reacts faster with CO. It is known that in the presence of UV, since the electron density increases, oxygen ions, O2−, at the surface of the ZnO can attract electrons and form more reactive ions such as O− and O2−,28 that can result in higher sensitivity to CO at room temperature. This phenomena was previously observed for TiO2 NPs too.28
Time transient behaviour of the devices was investigated by recording the sensitivity of the device to different percentages of CO in the environment, as displayed in Fig. 4c. The sensor shows a clear response to CO concentration as low as 1% (the lowest concentration change achievable with our measurement setup). Moreover, as shown in the Fig. 4c and the inset, the device follows any step like change in the concentration of the CO in the test chamber and returns to its value when the CO concentration is increased back to the initial CO value.
4. Conclusion
In this work, we have demonstrated sketching of low cost and ubiquitous sensors based on synthesized ZnO nanoparticle (average size 7–8 nm) inks on paper. Electrodes were sketched using carbon calligraphy ink and the ZnO layer was deposited using a painting brush as well as a custom-made stamp on a regular copy paper. These devices show more than three orders of magnitude enhancement in conductivity when exposed to 1220 μW cm−2 UV irradiation with a 6 s and 3 s response and recovery times to a 30 s pulse of UV light, respectively. Moreover, unlike the results reported so far, our sensors show strong response to CO at room temperature and can detect low concentrations of CO (as small as 1% in air). It was also observed that applying UV irradiation results in not only faster but also much more sensitive response to CO due to formation of more reactive oxygen ions at the surface of ZnO. This work highlights much higher potential for simple sketching of nanoelectronic sensors from synthesized conductive and semiconductive inks for fabrication of functional sensors of UV and CO and can pave the way for future ubiquitous sensors.Acknowledgements
The authors would like to acknowledge the financial support of Natural Sciences and Engineering Research Council (NSERC) of Canada and Canada Foundation for Innovation (CFI). We would like to thank Saeideh Ebrahimi Takalloo for her help in the preparation of the calligraphy ink.References
- J. A. Rogers, et al., Materials and mechanics for stretchable electronics, Science, 2010, 327, 1603–1607 CrossRef CAS PubMed.
- J. P. Rolland and D. A. Mourey, Paper as a novel material platform for devices, MRS Bull., 2013, 38, 299–305 CrossRef.
- D. Tobjörk and R. Österbacka, Paper electronics, Adv. Mater., 2011, 23, 1935–1961 CrossRef PubMed.
- M. C. Barr, et al., Direct monolithic integration of organic photovoltaic circuits on unmodified paper, Adv. Mater., 2011, 23, 3500–3505 CrossRef CAS PubMed.
- A. C. Siegel, et al., Foldable printed circuit boards on paper substrates, Adv. Funct. Mater., 2010, 20, 28–35 CrossRef CAS.
- A. VithaláGhule, Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study, Green Chem., 2006, 8, 1034–1041 RSC.
- A. J. Gimenez, et al., ZnO-Paper Based Photoconductive UV Sensor, J. Phys. Chem. C, 2010, 115, 282–287 Search PubMed.
- N. Mohseni Kiasari and P. Servati, Dielectrophoresis-Assembled ZnO Nanowire Oxygen Sensors, IEEE Electron Device Lett., 2011, 32, 982–984 CrossRef.
- N. M. Kiasari, et al., Room temperature ultra-sensitive resistive humidity sensor based on single zinc oxide nanowire, Sens. Actuators, A, 2012, 182, 101–105 CrossRef PubMed.
- C. Nützenadel, et al., Critical size and surface effect of the hydrogen interaction of palladium clusters, Eur. Phys. J. D, 2000, 8, 245–250 CrossRef.
- H. Gullapalli, V. S. Vemuru, A. Kumar, A. Botello-Mendez, R. Vajtai, M. Terrones, S. Nagarajaiah and P. M. Ajayan, Flexible piezoelectric ZnO–paper nanocomposite strain sensor, Small, 2010, 6, 1641–1646 CrossRef CAS PubMed.
- C. Pacholski, et al., Self-Assembly of ZnO: From Nanodots to Nanorods, Angew. Chem., Int. Ed., 2002, 41, 1188–1191 CrossRef CAS.
- J. R. Swider, et al., Characterization of Chinese ink in size and surface, J. Cult. Herit., 2003, 4, 175–186 CrossRef.
- F. Shan and Y. Yu, Band gap energy of pure and Al-doped ZnO thin films, J. Eur. Ceram. Soc., 2004, 24, 1869–1872 CrossRef CAS.
- K. J. Chen, et al., Optoelectronic characteristics of UV photodetector based on ZnO nanowire thin films, J. Alloys Compd., 2009, 479, 674–677 CrossRef CAS PubMed.
- T. H. Moon, et al., The fabrication and characterization of ZnO UV detector, Appl. Surf. Sci., 2005, 240, 280–285 CrossRef CAS PubMed.
- C. Soci, et al., ZnO nanowire UV photodetectors with high internal gain, Nano Lett., 2007, 7, 1003–1009 CrossRef CAS PubMed.
- Q. Li, et al., Adsorption and desorption of oxygen probed from ZnO nanowire films by photocurrent measurements, Appl. Phys. Lett., 2005, 86, 123117 CrossRef PubMed.
- Y. Li, et al., High-performance UV detector made of ultra-long ZnO bridging nanowires, Nanotechnology, 2009, 20, 045501 CrossRef PubMed.
- F. Fang, et al., UV and humidity sensing properties of ZnO nanorods prepared by the arc discharge method, Nanotechnology, 2009, 20, 245502 CrossRef CAS PubMed.
- J. Cheng, et al., UV Photoresponse and Field Emission Properties of ZnO Single Crystal Microtubes, Ferroelectrics, 2009, 382, 68–75 CrossRef CAS.
- S. J. Chang, et al., Highly sensitive ZnO nanowire CO sensors with the adsorption of Au nanoparticles, Nanotechnology, 2008, 19, 175502 CrossRef PubMed.
- J. Chang, et al., The effects of thickness and operation temperature on ZnO: Al thin film CO gas sensor, Sens. Actuators, B, 2002, 84, 258–264 CrossRef CAS.
- R. K. Joshi, et al., Au decorated zinc oxide nanowires for CO sensing, J. Phys. Chem. C, 2009, 113, 16199 CAS.
- V. V. Sysoev, et al., Toward the nanoscopic “electronic nose”: hydrogen vs. carbon monoxide discrimination with an array of individual metal oxide nano-and mesowire sensors, Nano Lett., 2006, 6, 1584–1588 CrossRef CAS PubMed.
- T. Barry and F. Stone, The reactions of oxygen at dark and irradiated zinc oxide surface, Proc. R. Soc. London, Ser. A, 1960, 255, 124–144 CrossRef CAS.
- N. M. Kiasari, et al., Environmental Gas and Light Sensing Using ZnO Nanowires, IEEE Trans. Nanotechnol., 2014, 13, 368–374 CrossRef CAS.
- T.-Y. Yang, et al., UV enhancement of the gas sensing properties of nano-TiO2, Rev. Adv. Mater. Sci., 2003, 4, 48–54 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |