Sulfur-impregnated MWCNT microball cathode for Li–S batteries†
Jin-Hoon Choiacd,
Cho-Long Leea,
Kyu-Sung Parkb,
Sung-Moo Joc,
Dae-Soon Limd and
Il-Doo Kim*a
aDepartment of Materials Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon 305-701, Republic of Korea. E-mail: idkim@kaist.ac.kr
bTexas Materials Institute, The University of Texas at Austin, Austin, TX 78712, USA
cCarbon Convergence Materials Research Division, Korea Institute of Science and Technology, Seoul, 136-791, Republic of Korea
dDepartment of Materials Science and Engineering, Korea University, Seoul, 136-713, Republic of Korea
First published on 20th March 2014
Abstract
We report a facile synthesis, via an electrostatic spray route, of MWCNT microballs that are used as highly porous templates for effective sulfur impregnation. Mesoporous MWCNT microballs with a three-dimensional interpenetrating network structure offer a promising solution to not only maximize the energy density but also guarantee high power capability. Furthermore, the high specific surface area (175.24 m2 g−1) of the microballs provides large pore volumes suitable for effective sulfur impregnation. A sulfur-impregnated MWCNT cathode showed superior electrochemical cell performance for long-term and high rate capability. In particular, sulfur-impregnated MWCNT microball based electrodes have a significant advantage to secure a mechanically robust carbon structure with better electrical contact during cycling.
Introduction
Li–S batteries have attracted great attention owing to their high specific capacity (1675 mA h g−1), which is based on the electrochemical reaction of 16Li + S8 → 8Li2S, and high specific energy density (2600 W h kg−1 or 2800 W h L−1), which is suitable for next generation energy storage devices. Elemental sulfur is also abundant, non-toxic and cheap.1–3 However, despite these advantages, some major challenges still remain in developing a practical Li–S battery. The first hurdle is that sulfur and its solid reduction products (Li2S and Li2S2) are electrically insulating.4 Second, the polysulfide, Li2Sn (3 ≤ n ≤ 6), intermediates formed during charge/discharge dissolve in organic electrolytes.5,6 Over the years, there have been many efforts to solve these challenges by encapsulating sulfur with conducting materials such as porous carbon,7,8 carbon nanotube/fiber,9–11 graphene12–14 and conducting polymer.15,16 Among these various conducting materials, carbon nanotubes (CNT) have not been actively studied for Li–S batteries because it is difficult to use CNTs to form a 3-dimensional (3D) conducting network suitable to cover sulfur fully and uniformly. There are some simple approaches to form CNT paper electrodes17 or to grow vertically aligned CNT films using a chemical vapor deposition process.18 However, there have not been any serious attempts to design a 3D CNT nanocomposite structure to improve sulfur utilization and to confer high-rate capability in Li–S batteries.Here, we propose a unique 3D porous structure composed of CNTs. It is possible that porous nanostructures will provide a solution to the critical issue associated with sulfur loading level and sulfur utilization.1,2 A mesoporous architecture with a 3D interpenetrating network provides fast Li+-ion transport pathways and large pore volumes, making this architecture suitable for a large amount of sulfur loading.19 Therefore, developing mesoporous electrode architectures with optimal electrical connections should be a promising solution to not only maximize the energy density but also guarantee the high power capability; at the same time, the mechanical–chemical stability of the electrode should also be assured for long-term durability of the Li–S batteries. With these considerations, we have developed a simple and effective way to form a sulfur impregnated CNT composite and to improve the electrochemical utilization of the sulfur within the stable framework.
Fig. 1 shows the electrostatic spray (E-spray) coating method used to form spherical floccus-like multi-walled CNT (MWCNT) aggregates as hosting substrates and the following melt-diffusion process with sulfur. Through these processes, it was easily possible to synthesize the sulfur and MWCNT composite as an active material. During the E-spray process, the organic binder included in the MWCNT solution was self-clustered to form compact spheres with uniform size distribution. Next, highly mesoporous MWCNT sphere (MMS) particles were achieved using the binder burnout process, which eliminates the electrically insulating binder. For the impregnation of sulfur, we used the melt-diffusion strategy, which was previously adopted in a study of CMK-3 sulfur composites.1 Especially, almost all the initial MMS particles were preserved during the entire process.
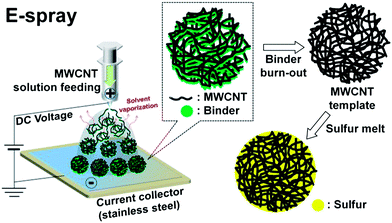 | ||
| Fig. 1 Schematic illustration of sequential fabrication steps for the sulfur–MMS composite particles. | ||
Experimental
Synthesis of MMS and sulfur–MMS composite
The commercial bulk sulfur powder (100 mesh powder, purified by sublimation, Sigma-Aldrich Co., Ltd., USA) and 6 wt% MWCNTs–polyvinyl acetate (1/1 wt%) dispersion in isopropyl alcohol (IPA) (CNTol, World-Tube Co. Ltd., Korea) were used in this work. The mesoporous spherical particles, consisting of MWCNTs (MMS), were prepared by the following procedure. To prepare a starting solution for the E-spray process, 6 wt% CNTol was diluted with IPA at a weight ratio of 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.97 (MWCNTs–polyvinyl acetate:IPA). Then, the diluted solution was agitated for 30 minutes under ultrasonic vibration and subsequently loaded into a plastic syringe. The E-spray coating of the 3 wt% CNTol was carried out with a feeding rate of 17–20 μL min−1 on a stainless steel (SUS) plate that was vertically positioned 20 cm away from a grounded collector. A voltage of 15–17 kV was applied from a high-voltage DC power supply to the tip of a SUS needle (23 gauge) connected to the syringe. After the E-spray process, the binder (polyvinyl acetate)-included MMS particles were collected on the SUS plate and immediately put into an electrical furnace (Vulcan 3-550, Ney) to burn out the binder. The heat treatment temperature was increased from room temperature to 400 °C at 5 °C min−1 and kept at 400 °C for 30 minutes under air atmosphere to ensure the complete removal of the binder. Then, binder-free MMS was obtained from the SUS plate by raking the plate surface with a razor blade.
0.97 (MWCNTs–polyvinyl acetate:IPA). Then, the diluted solution was agitated for 30 minutes under ultrasonic vibration and subsequently loaded into a plastic syringe. The E-spray coating of the 3 wt% CNTol was carried out with a feeding rate of 17–20 μL min−1 on a stainless steel (SUS) plate that was vertically positioned 20 cm away from a grounded collector. A voltage of 15–17 kV was applied from a high-voltage DC power supply to the tip of a SUS needle (23 gauge) connected to the syringe. After the E-spray process, the binder (polyvinyl acetate)-included MMS particles were collected on the SUS plate and immediately put into an electrical furnace (Vulcan 3-550, Ney) to burn out the binder. The heat treatment temperature was increased from room temperature to 400 °C at 5 °C min−1 and kept at 400 °C for 30 minutes under air atmosphere to ensure the complete removal of the binder. Then, binder-free MMS was obtained from the SUS plate by raking the plate surface with a razor blade.
The sulfur–MMS composite was prepared using a melt-diffusion strategy. In a crucible with a cover, sulfur and the MMS with ethanol were mixed by ultrasonic agitation for 30 minutes. The weight ratios of sulfur![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMS were adjusted to be 7
MMS were adjusted to be 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 8
3 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Next, the solutions of sulfur and MMS dispersion in ethanol were dried at 80 °C for 2 hours under air atmosphere. To insert sulfur into the MMS particles, the mixed composite samples were heated at 155 °C for 8 hours in an electrical tube furnace (Ajeon Heating Industrial Co. Ltd., Korea) with a constant flow of Ar gas; the heating rate was 5 °C min−1.
2. Next, the solutions of sulfur and MMS dispersion in ethanol were dried at 80 °C for 2 hours under air atmosphere. To insert sulfur into the MMS particles, the mixed composite samples were heated at 155 °C for 8 hours in an electrical tube furnace (Ajeon Heating Industrial Co. Ltd., Korea) with a constant flow of Ar gas; the heating rate was 5 °C min−1.
Microstructural characterization
The microstructural evolutions of the binder-included MMS, the binder-free MMS and the sulfur–MMS composite were observed by using a scanning electron microscope (Field Emission SEM, Magellan400, FEI) and a transmission electron microscope (FE-TEM 300 KV, Tecnai). EDS for the elemental analysis and mapping was carried out on the TEM and SEM. The specific surface area and pore structural parameters of the MMS were determined by BET measurements (ASAP2020, Micrometrics). To compare the thermal behavior of the sulfur–MMS particles with different levels of sulfur loading (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 8
3 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), TGA and DTG were carried out under Air flow of 30 mL min−1 using a Thermogravimetric Analyzer (TG 209 F3, NETZSCH) operated at temperatures up to 800 °C at a heating rate of 10 °C min−1.
2), TGA and DTG were carried out under Air flow of 30 mL min−1 using a Thermogravimetric Analyzer (TG 209 F3, NETZSCH) operated at temperatures up to 800 °C at a heating rate of 10 °C min−1.
Electrochemical characterization
The pristine sulfur cathode and the sulfur–MMS composite electrode were prepared by mixing sulfur with Super P carbon black and polyvinylidene fluoride (PVDF) at weight ratios of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 84
10 and 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, respectively. A small amount of n-methyl-2-pyrrolidone (NMP) was added in the course of mechanical mixing until the active materials showed the right viscosity. The mixed slurry was casted on Al foil and subsequently dried at 50 °C overnight in a convection oven. The morphology of the slurry-casted sulfur–MMS composite electrode is shown in Fig. S6.† Electrochemical performances of the pristine sulfur cathode and the sulfur–MMS composite electrodes were evaluated with coin half-cells (2032, Hohsen). An Li-metal foil was used as the counter electrode and 1 M LiTFSI in a 1
8, respectively. A small amount of n-methyl-2-pyrrolidone (NMP) was added in the course of mechanical mixing until the active materials showed the right viscosity. The mixed slurry was casted on Al foil and subsequently dried at 50 °C overnight in a convection oven. The morphology of the slurry-casted sulfur–MMS composite electrode is shown in Fig. S6.† Electrochemical performances of the pristine sulfur cathode and the sulfur–MMS composite electrodes were evaluated with coin half-cells (2032, Hohsen). An Li-metal foil was used as the counter electrode and 1 M LiTFSI in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (by volume) of TEGDME
1 mixture (by volume) of TEGDME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIOX (Soulbrain Co., Ltd., Korea) with 0.2 M LiNO3 was used as the electrolyte. The separator was a Celgard 2325 (25 μm thick). The cells were galvanostatically charged and discharged between 1.5 and 3.0 V at various current densities. The electrochemical impedance spectroscopy (Si 1260, Solatron) of the pristine sulfur and sulfur–MMS composite cathodes were measured in a frequency range from 100 kHz to 10 mHz with AC voltage amplitude of 5 mV.
DIOX (Soulbrain Co., Ltd., Korea) with 0.2 M LiNO3 was used as the electrolyte. The separator was a Celgard 2325 (25 μm thick). The cells were galvanostatically charged and discharged between 1.5 and 3.0 V at various current densities. The electrochemical impedance spectroscopy (Si 1260, Solatron) of the pristine sulfur and sulfur–MMS composite cathodes were measured in a frequency range from 100 kHz to 10 mHz with AC voltage amplitude of 5 mV.
Results and discussions
Fig. 2 shows the microstructural evolution of the self-aggregated MMS and sulfur–MMS composite particles. Before the binder burnout process, the individual MWCNTs were aggregated to a spherical shape with a uniform size distribution of 3–6 μm (Fig. 2a). Fig. 2b provides a magnified SEM image of the aggregated MMCNT spherical particles; this image shows that the particles were composed of tightly packed individual MMCNTs with pores filled with an organic binder. Fig. 2c and d show the highly porous morphology of the self-aggregated MMS particles after the binder burnout process. This result was also confirmed by Brunauer–Emmett–Teller (BET) measurements (Fig. S1†); the spheres also exhibited high specific surface area of 175.24 m2 g−1.We initially prepared two sets of sulfur–MMS composites whose weight ratios were 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 8
3 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (sulfur
2 (sulfur![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMS). The sulfur content of the composites was confirmed by Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTG) (Fig. S2†). The sulfur–MMS (8/2) composite showed severe aggregation of sulfur, in which the MMS particles were partially buried (Fig. S3†). On the contrary, molten sulfur flowed perfectly into the MMS through the pores in the case of the sulfur–MMS (7/3) sample (Fig. 2e); this flow was confirmed by Transmission Electron Microscopy (TEM), with a TEM image provided in Fig. 2f. Additionally, the existence of sulfur in the MMS was verified again by Energy Dispersive Spectrometer (EDS) analysis (Fig. S4 and Table S1†). To confirm the homogenous atomic distributions of sulfur and carbon in the sulfur–MMS composite, EDS mapping analysis was carried out (Fig. 2g). Each element was uniformly dispersed throughout the whole region of the particle without any local segregation.
MMS). The sulfur content of the composites was confirmed by Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTG) (Fig. S2†). The sulfur–MMS (8/2) composite showed severe aggregation of sulfur, in which the MMS particles were partially buried (Fig. S3†). On the contrary, molten sulfur flowed perfectly into the MMS through the pores in the case of the sulfur–MMS (7/3) sample (Fig. 2e); this flow was confirmed by Transmission Electron Microscopy (TEM), with a TEM image provided in Fig. 2f. Additionally, the existence of sulfur in the MMS was verified again by Energy Dispersive Spectrometer (EDS) analysis (Fig. S4 and Table S1†). To confirm the homogenous atomic distributions of sulfur and carbon in the sulfur–MMS composite, EDS mapping analysis was carried out (Fig. 2g). Each element was uniformly dispersed throughout the whole region of the particle without any local segregation.
The electrochemical properties of the sulfur–MMS composite electrode were investigated, with results as shown in Fig. 3. For comparison and to match with the carbon content of the sulfur–MMS electrode, a pristine sulfur electrode having 30 wt% of carbon black (Super P) and 10 wt% of PVDF binder was also prepared using the conventional slurry casting method. The carbon content of the slurry-casted sulfur–MMS electrode (sulfur–MMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Super P
Super P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVDF = 84
PVDF = 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt%) was 33.2 wt%, so the mass of sulfur in the sulfur–MMS composite electrode was 58.8% of the total weight of the electrode.
8 wt%) was 33.2 wt%, so the mass of sulfur in the sulfur–MMS composite electrode was 58.8% of the total weight of the electrode.
Fig. 3a shows the charge/discharge voltage curves of the sulfur–MMS composite electrode (sulfur loading level = 0.45 mg cm−2) during galvanostatic cycling at 0.5 C. Two typical plateaus at 2.4 and 2.1 V for discharging were identified, corresponding to the formation of long-chain liquid polysulfides (Li2Sx, 4 < x < 8) and solid Li2S2/Li2S, respectively.20 To further investigate the cyclability and kinetic properties of the sulfur–MMS composite electrode, a long-term cycling test for 100 cycles at 0.5 C was carried out (Fig. 3b). A capacity of 548.5 mA h g−1 was maintained after 100 cycles. The capacity retention of the sulfur–MMS was more stable than those of the pristine sulfur electrodes with sulfur loading levels of 0.2 and 0.4 mg cm−2; these two pristine sulfur electrodes show continuous capacity fading for 100 cycles and drop to value of 459.8 and 322.9 mA h g−1, respectively. It is worth noting that the value of 322.9 mA h g−1 of the pristine sulfur electrode is 58.9% of that of the sulfur–MMS composite electrode with similar areal sulfur loading level. Without stable carbon structures, a large amount of sulfur in the electrode could not be fully and reversibly charged/discharged.
Fig. 3c shows the discharge capacities of the pristine sulfur and sulfur–MMS composite electrodes (0.9 mg cm−2) at different current densities. During the initial five preconditioning cycles at 0.05 C, the discharge capacities gradually faded and stabilized at 0.1 C. The reversible capacities at this points, each sample reached values of 682.0 (sulfur–MMS) and 609.8 (pristine sulfur) mA h g−1. As the current rate increased from the 0.1 C to 2 C, the rate capability of the sulfur–MMS composite electrode was excellent, while the pristine sulfur electrode with the same carbon loading content (30 wt%) showed poor current responses. The pristine sulfur electrode was unable to sustain C-rates higher than 1 C, while the sulfur–MMS composite electrode was able to deliver 446.9 mA h g−1 even at 2 C. After the 2 C-rate cycling, the capacity of the sulfur–MMS composite electrode fully recovered when the current density was reduced to the 0.05 C-rate, which indicates that there was no irreversible electrode degradation during high C-rate cycling. In comparison, capacity fading of the pristine sulfur electrode was found to continuously occur even after the recovery cycling at 0.05 C. These results confirm the stable electrochemical activity of the polysulfides in the sulfur–MMS composite electrode.
To emphasize the high rate capability of the sulfur–MMS composite electrode, the discharge voltage curves, drawn as a function of the applied rates, a provided in Fig. 3d. In the sulfur–MMS electrode, the capacity decrease mainly happens in the 2.1 V plateau region which involves solid Li2S2/Li2S nucleation and growth reactions. In contrast, the pristine sulfur electrode shows a major polarization increase and capacity drop in the 2.4 V plateau region as well as partly in the 2.1 V plateau region. This difference clearly suggests that the carbon structure in the pristine sulfur electrode cannot provide sufficient electrons to hold the charged sulfur properly. This hypothesis was verified using a morphology analysis of the cycled cathodes.
Fig. 4 shows SEM images of the cycled (0.5 C for 100 cycles) sulfur–MMS and the pristine sulfur electrodes before and after washing the sulfur out. In the sulfur–MMS electrode, it was possible to observe overflow polysulfide species from the MMS structure, which suggests that the sulfur content needs to be further reduced so as not to electrically isolate part of the active sulfur material.
 | ||
| Fig. 4 SEM images of (a) the sulfur–MMS and (b) the pristine sulfur electrodes after cycling. Inset images show the local structures after washing sulfur out of the electrodes. | ||
However, it is clear that the MMS carbon structure remains intact during the charge/discharge cycles. In an Li–S battery, the final charged form is elemental sulfur (α-phase; density = 2.07 g cm−3); the final discharged form is Li2S (density = 1.66 g cm−3). In addition, the intermediate discharge products are liquid, so there are repeated volume changes during cycling. In the case of the pristine electrode, the volume change mechanically degraded the carbon structure in the electrode. A large portion of the carbon is detached from the current collector. Therefore, our 3D composite electrode strategy has a significant advantage in attempts to secure a mechanically robust carbon structure with better electrical contact (Fig. S5†). Moreover, MMS frameworks have a more open electrode structure with high surface area, which may have some benefits for electrolyte wetting and Li+-ion transport.
Conclusions
In summary, we have proposed a sulfur and carbon composite structure with a self-aggregated MWCNTs network, which is very effective for the electrochemical utilization of the sulfur. Sulfur-embedded MWCNTs particles were successfully synthesized via the E-spray process and subsequent melt-diffusion of sulfur into mesoporous MWCNTs spheres. The sulfur–MMS composite electrode can provide the following characteristics: (i) efficient and rapid pathways for Li+-ion and electron transport to overcome the insulating nature of solid active materials (S, Li2S2 and Li2S), (ii) short solid-state diffusion lengths with large surface area to achieve high power and energy density, (iii) mechanically stable 3D carbon frameworks to overcome the volume change of active materials during cycling. Furthermore, our electrode fabrication process is facile and efficient, so it can easily applied to other nanostructured Li–S cathode materials with desirable morphologies.Acknowledgements
This work was supported by the Center for Integrated Smart Sensors funded by the Ministry of Education, Science and Technology as Global Frontier Project (CISS-2012M3A6A6054188). This work was also supported by a National Research Foundation of Korea grant funded by the Engineering Research Center (2012-0001175).Notes and references
- X. L. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500 CrossRef CAS PubMed.
- A. Manthiram, Y. Z. Fu and Y. S. Su, Acc. Chem. Res., 2013, 46, 1125 CrossRef CAS PubMed.
- H. Yamin and E. Peled, J. Power Sources, 1983, 9, 281 CrossRef CAS.
- Y. Yang, G. Y. Zheng, S. Misra, J. Nelson, M. F. Toney and Y. Gui, J. Am. Chem. Soc., 2012, 134, 15387 CrossRef CAS PubMed.
- H. Yamin, A. Gorenshtein, J. Penciner, Y. Sternberg and E. Peled, J. Electrochem. Soc., 1988, 135, 1045 CrossRef CAS.
- R. Elazari, G. Salitra, Y. Talyosef, J. Grinblat, C. Scordilis-Kelley, A. Xiao, J. Affinito and D. Aurbach, J. Electrochem. Soc., 2010, 157, A1131 CrossRef CAS.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904 CrossRef CAS PubMed.
- B. Zhang, X. Qin, G. R. Li and X. P. Gao, Energy Environ. Sci., 2010, 3, 1531 CAS.
- L. C. Yin, J. L. Wang, J. Yang and Y. N. Nuli, J. Mater. Chem., 2011, 21, 6807 RSC.
- G. Y. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462 CrossRef CAS PubMed.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288 CrossRef CAS PubMed.
- S. Evers and L. F. Nazar, Chem. Commun., 2012, 48, 1233 RSC.
- N. W. Li, M. B. Zheng, H. L. Lu, Z. B. Hu, C. F. Shen, X. F. Chang, G. B. Ji, J. M. Cao and Y. Shi, Chem. Commun., 2012, 48, 4106 RSC.
- H. L. Wang, Y. Yang, Y. Y. Liang, J. T. Robinson, Y. G. Li, A. Jackson, Y. Cui and H. J. Dai, Nano Lett., 2011, 11, 2644 CrossRef CAS PubMed.
- Y. Z. Fu and A. Manthiram, RSC Adv., 2012, 2, 5927 RSC.
- L. F. Xiao, Y. L. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. M. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176 CrossRef CAS PubMed.
- Y. S. Su and A. Manthiram, Chem. Commun., 2012, 48, 8817 RSC.
- S. Dorfler, M. Hagen, H. Althues, J. Tubke, S. Kaskel and M. J. Hoffmann, Chem. Commun., 2012, 48, 4097 RSC.
- H. G. Zhang, X. D. Yu and P. V. Braun, Nat. Nanotechnol., 2011, 6, 277 CrossRef CAS PubMed.
- S. E. Cheon, K. S. Ko, J. H. Cho, S. W. Kim, E. Y. Chin and H. T. Kim, J. Electrochem. Soc., 2003, 150, A796 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (S1) BET analysis of MMS, (S2) TGA/DTG measurement, (S3) SEM images, (S4) EDS spectrum, (Table S1) quantitative elemental information, (S5) electrochemical impedance spectra of sulfur–MMS composites, (S6) SEM image of pristine sulfur electrode. See DOI: 10.1039/c4ra01919a |
| This journal is © The Royal Society of Chemistry 2014 |


