A fast-response, highly sensitive and selective fluorescent probe for the ratiometric imaging of hydrogen peroxide with a 100 nm red-shifted emission
Caiyun Liua,
Changxiang Shaob,
Huifang Wub,
Bingpeng Guob,
Baocun Zhu*b and
Xiaoling Zhang*a
aKey Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: zhangxl@bit.edu.cn; Fax: +86-10-88875298; Tel: +86-10-88875298
bSchool of Resources and Environment, University of Jinan, Jinan 250022, China. E-mail: lcyzbc@163.com
First published on 13th March 2014
Abstract
Recently, growing attention has been paid to the accurate determination of hydrogen peroxide (H2O2) for elucidating its detailed biological function in physiology and pathology. A fluorescence method with the help of a fluorescent probe is the preferred technique for in situ visualization of biologically important species in vivo, even in single living cells. In the present manuscript, we developed a simple, fast response and highly selective fluorescent probe (1) with a receptor of the boronate moiety for the ratiometric imaging of H2O2 in living cells. Probe 1 could quantifiably detect H2O2 in the range of 18–540 μM by a ratiometric fluorescence spectroscopy method with a detection limit of 4 μM. Importantly, probe 1 exhibited 81 nm red-shifted absorption spectra accompanied by the color changes from colorless to yellow, and 100 nm red-shifted emission spectra upon addition of H2O2. Thus, 1 can serve as a “naked-eye” probe for H2O2. Preliminary bioimaging application and low cytotoxicity investigations further demonstrated that the proposed probe would be of great benefit to biomedical researchers for investigating the detailed biological function of H2O2 in biological systems.
1. Introduction
Hydrogen peroxide (H2O2), a major reactive oxygen species (ROS) produced in living matrixes, is attracting growing attention for its diverse functions in physiology and pathology.1–4 Hydrogen peroxide is a newly recognized second messenger in cellular signal transduction and can indicate the intracellular oxidant levels.5–7 A regular level of H2O2 plays important roles in physiology, aging, and disease in living organisms.8,9 Abnormal production or accumulation in cellular levels of H2O2 has been linked to DNA damage, mutation, and genetic instability.6,10 Thus, the development of accurate assays for H2O2 is of great importance for elucidating the detailed biological function of H2O2 in living systems.Fluorescence technique with the help of fluorescent probe is the preferred method for in situ visualization of biologically important species in living systems owing to its various advantages such as simplicity, sensitivity, non-invasiveness, and good biological compatibility.11,12 Despite advances in the development of fluorescent probes for H2O2, the ratiometric fluorescent probes for H2O2 are rather few even such probes can eliminate numerous ambiguities resulting from the localization of the probe, changes of environment around the probes, excitation and emission efficiency.4,13–18 Additionally, the ratiometric fluorescent probe should exhibit a large emission wavelength shift (above 80 nm) for enhancing resolution in the ratiometric bioimaging.4,19,20 As far as we know, only three such probes for H2O2 have been reported so far. One example reported by Jiang et al. showed a large bathochromic shift of 105 nm in the recognition of H2O2 and this property can be achieved only in the presence of surfactant solution.18 Kumar et al. presented a ratiometric fluorescent probe exhibited an 82 nm blue-shifted emission in the presence of H2O2.21 This probe is prone to be interfered with pH for it employs the twisted intramolecular charge transfer (TICT) mechanism based the charge transfer from the nitrogen atom of the dimethylamino moiety (donor) to the imino moiety (acceptor). Very recently, we developed a ratiometric fluorescent probe for H2O2 with a large emission shift of 132 nm and this probe possesses a defect of shorter excitation wavelength (λex = 380 nm).4 Although the three fluorescent probes have made great progress in the ratiometric determination of H2O2, more effective probes with fast-response, high sensitivity and selectivity, and practicability, especially ratiometric imaging in living cells still need to be developed.
Recently, based on the internal charge transfer (ICT) mechanism, we developed a series of ratiometric fluorescent probes employing diversified fluorophores.22–24 For example, 4-hydroxynaphthalimide was selected firstly as the fluorescence reporter group in the design of ratiometric fluorescent probes.24 A propargyl protecting 4-hydroxynaphthalimide exhibited 73 nm red-shifted emission spectra upon addition of palladium species, and thus, 4-hydroxynaphthalimide is a potential fluorophore in the construction of ratiometric fluorescent probes with a large emission wavelength shift. In continuation of our research work, we herein designed and synthesized an ICT-based ratiometric fluorescent probe (Scheme 1, 1) for H2O2 with apparent properties such as fast-response (within 10 min), high sensitivity (a detection limit of 4 μM) and selectivity, a large emission shift of 100 nm, visual and ratiometric detection, and ratiometric imaging in living cells.
During the development of fluorescent probes for H2O2, Chang et al. pioneered a novel boronate-based fluorescent probe for the high selective determination of H2O2.25,26 A receptor of boronate moiety was widely adopted in the design of probes for H2O2.6,12 This strategy inspired us to design highly selective fluorescent probes for H2O2 by the adoption of boronate moiety. To achieve the ratiometric measurement, 4-hydroxynaphthalimide was selected as the fluorescence reporter group for it possesses excellent ICT structure and desirable photophysical properties.24 We hypothesize that the reaction of probe 1 with H2O2 triggers the cleavage of a boronate-based protecting group, and as a result, restores the green fluorescence of 4-hydroxynaphthalimide 2. The reaction mechanism of 1 with H2O2 is shown in Scheme 2.
2. Experimental
2.1 Materials and instrumentations
4-Hydroxy-1,8-naphthalimide was prepared according our previous work.24 All other chemicals used in this paper were obtained from commercial suppliers and used without further purification. Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co.) was used for column chromatography. 1H-NMR was recorded on a Bruker AV-400 spectrometer with chemical shifts reported as ppm (in CDCl3, TMS as internal standard). Electrospray ionization (ESI) mass spectra were measured with an LC-MS 2010A (Shimadzu) instrument. Absorption spectra were recorded on UV-3101PC spectrophotometer. Fluorescence emission spectra were measured on Perkin-Elmer Model LS-55. All pH measurements were made with a Sartorius basic pH-meter PB-10.2.2 Synthesis of probe 1
To a solution of 4-hydroxy-1,8-naphthalimide 2 (269 mg, 1 mmol) in CH3CN (30 mL) were added K2CO3 (276 mg, 2 mmol) and 4-(bromomethyl)benzene boronic acid pinacol ester (297 mg, 1 mmol). The reaction mixture was heated to reflux overnight. After removal of solvent, the residues were purified by silica gel column chromatography using dichloromethane as eluent to afford pure product. 1H-NMR (400 MHz, CDCl3) δ (*10−6): 0.971 (t, J = 7.4 Hz, 3H), 1.358 (s, 12H), 1.415–1.472 (m, 2H), 1.669–1.745 (m, 2H), 4.167 (t, J = 7.4 Hz, 2H), 5.389 (s, 2H), 7.093 (d, J = 8.4 Hz, 1H), 7.519 (d, J = 8.0 Hz, 2H), 7.709 (t, J = 8.0 Hz, 1H), 7.882 (d, J = 8.0 Hz, 2H), 8.526 (d, J = 8.4 Hz, 1H), 8.604–8.627 (m, 2H). ESI-MS calcd for C29H33BNO5 [M + H]+ 486.2, found 486.2.2.3 Determination of the detection limit
The detection limit was calculated based on the fluorescence titration. In the absence of H2O2, the fluorescence emission spectrum of probe 1 was measured by five times and the standard deviation of blank measurement was achieved. To gain the slope, the ratio of the fluorescence intensity at 546 nm to the fluorescence intensity at 446 nm (F546/F446) was plotted as a concentration of H2O2. So the detection limit was calculated with the following equation:| Detection limit = 3σ/k. |
2.4 Cell culture and fluorescence imaging
RAW264.7 macrophage cells (gifted from the center of cells, Peking Union Medical College) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS) supplemented with 100 U mL−1 of penicillin and 100 μg mL−1 streptomycin at 37 °C under a humidified atmosphere containing 5% CO2. RAW264.7 macrophage cells were seeded in a 96-well plate at a density of 104 cells per well in culture media. After 24 h, they were incubated with 5 μM probe 1 in culture media for 20 min at 37 °C. Fluorescence imaging of living RAW264.7 macrophage cells was observed under an Olympus FV1000 confocal fluorescence microscope with excitation wavelength fixed at 405 nm. Then, the cells were incubated with 100 μM H2O2 for another 15 min and fluorescence imaging of living RAW264.7 macrophage cells was also observed under confocal fluorescence microscope with excitation wavelength fixed at 405 nm.2.5 Cytotoxicity assays
RAW264.7 macrophage cells were cultured in culture media (DMEM) in an atmosphere of 5% CO2 and 95% air at 37 °C. The cells were seeded into 96-well plates at a density of 3 × 103 cells per well in culture media, then 0, 5, 10, 25 and 50 μM (final concentration) probe 1 were added respectively. Next, the cells were incubated at 37 °C in an atmosphere of 5% CO2 and 95% air for 1 h. Finally, 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg mL−1) was added and were cultured for another 4 h, respectively.3. Results and discussion
3.1 Characteristic spectrum
It is reported that the appropriate balance between hydrophilicity and lipophilicity of probe is beneficial to the cell permeability and bioimaging in living systems. So, the determination of H2O2 with probe 1 was investigated under a mixture of ethanol and water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution containing phosphate buffered saline (PBS) (5 mM, pH 7.4).
5, v/v) solution containing phosphate buffered saline (PBS) (5 mM, pH 7.4).
Firstly, we assessed the spectroscopic properties of free probe 1 under the above analytical conditions. As shown in Fig. 1a and d, the solution of free probe 1 showed one major absorption peak at around 372 nm and fluorescence emission peak at around 446 nm. However, upon addition of H2O2 (500 μM), the maximum absorption peak exhibited an about 81 nm red shift and the color of the solution turned from colorless to yellow, and the maximum emission peak underwent a red shift to 546 nm. Thus, probe 1 can serve as a “naked-eye” indicator for H2O2. The considerable changes of absorption and fluorescence spectra might be ascribed to the cleavage of a boronate-based protecting group and the coinstantaneous production of stronger electron-push capacity of oxygen anion. These spectra of the reaction solution are in good agreement with ones of 4-hydroxy-1,8-naphthalimide reported by us.24 The results implied that the cleavage of boronate protecting 4-phenylmethanol moiety was induced by H2O2.
Then, to demonstrate the recognition mechanism of probe 1 for H2O2, the reaction of probe 1 and H2O2 was carried out under the above-mentioned analytical conditions. The green fluorescent reaction product was obtained and characterized to be compound 2 by 1H-NMR and ESI-MS. Therefore, combined with the previous conclusions by other groups,6,12 a possible mechanism was proposed as shown in Scheme 2.
3.2 Effects of reaction time on sensing H2O2
Response time is a fundamental parameter for most reaction-based probes. Then, the time required for the reaction of probe 1 and H2O2 at 25 °C was investigated. As shown in Fig. 2, the fluorescence intensity at 446 nm decreases with reaction time, and synchronously the fluorescence intensity at 546 nm increases with reaction time. The ratio of fluorescence intensities (F446/F546) decreases with reaction time and then levels off at reaction time greater than about 10 min. The result showed that the rate of the reaction of probe 1 with H2O2 is superior to those reported boronate-based fluorescent probes without additional reagents.4,6,12 That is to say, our proposed probe could provide a rapid analytical method for the detection of H2O2.3.3 Quantification of H2O2
In the absorption spectrum, the subsequent addition of H2O2 to the solution of probe 1 resulted in a gradual decrease of the maximum absorption peak centered at around 372 nm and a progressive increase of the maximum absorption peak centered at around 453 nm, and a well-defined isosbestic point at 402 nm is observed (Fig. 1a). Additionally, a good linearity between the absorbance ratio (A453/A372) and the concentrations of H2O2 in the range of 18 to 540 μM exhibited the determination of H2O2 would be carried out by the ratiometric measurement (Fig. 1b). Additionally, the detection limit by the naked-eye was estimated to be about 50 μM (Fig. 1c). In the fluorescence emission spectrum, the subsequent addition of H2O2 to the solution of probe 1 resulted in a gradual decrease of fluorescence peak centered at around 446 nm and a progressive increase of fluorescence peak centered at around 546 nm (Fig. 1d). In addition, a well-defined isoemission point at 520 nm is also observed (Fig. 1d). The production of isosbestic point and isoemission point distinctly demonstrated that a new species 4-hydroxy-1,8-naphthalimide 2 came into being. Moreover, there was a good linearity between the fluorescence intensity ratio (F546/F446) and the concentrations of H2O2 in the range of 18 to 540 μM with a detection limit of 4 μM (Fig. 1e). These results demonstrated that probe 1 could detect H2O2 qualitatively and quantitatively by ratiometric absorption and fluorescence methods with excellent sensitivity.3.4 Selectivity to H2O2
Then we examined the selectivity of probe 1 toward H2O2 under the above-mentioned analytical conditions. As shown in Fig. 3c, nearly no fluorescence intensity changes were observed in the presence of biological metal ions, anions, and other ROS including ClO−, O2−, ˙OH, TBHP, 1O2, NO, and ˙OtBu (Fig. 3c), which is ascribed to the adoption of a H2O2-specific receptor of boronate moiety. In addition, the effects of interference of the above-mentioned other analytes on monitoring H2O2 were investigated (Fig. 3d). The similar results were obtained in the absorption spectra (Fig. 3a and b). Thus, these results demonstrated that probe 1 possesses high selectivity toward H2O2 when present with other analytes.3.5 Bioimaging and cytotoxicity investigation
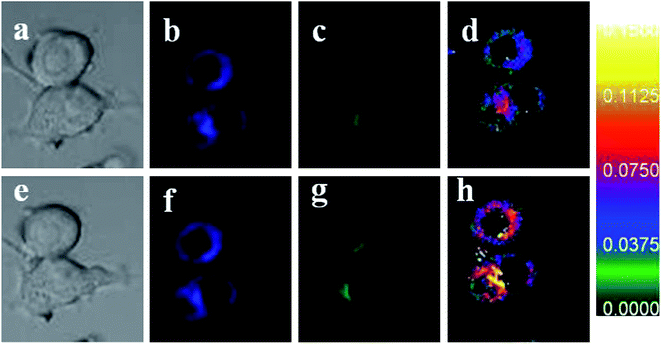
Next, we attempted to apply probe 1 for the ratiometric fluorescence imaging of H2O2 in living systems. Confocal fluorescence imaging in living RAW264.7 macrophage cells was carried out (Fig. 4.). An intense intracellular fluorescence of the cells incubated with probe 1 (5 μM) for 20 min demonstrated that probe 1 is cell-permeable (Fig. 4b and c). And then, 100 μM H2O2 was added to the above-mentioned cells for another 15 min. As expected, distinct changes of ratiometric fluorescence responses generated from green channel and blue channel in living cells were observed (Fig. 4d and h). These results revealed that probe 1 could be used for the ratiometric fluorescent imaging of H2O2 in living matrixes.Further, to estimate cytotoxicity of probe 1, we performed 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays in RAW264.7 macrophage cells with 5, 10, 25 and 50 μM probe 1 for 1 h, respectively. The experiment results were shown in Fig. 5. The obtained results exhibited the absorbances at 490 nm were in good agreement with the control experiment. This conclusion implied that our proposed probe was of low toxicity to cultured cells under the experimental conditions at the concentration of 5 μM for 35 min.
4. Conclusions
In conclusion, we have presented the design, synthesis and properties of an ICT-based ratiometric fluorescent probe 1 for H2O2 with a new design platform of benzyl protecting 4-hydroxynaphthalimide. Probe 1 exhibits high H2O2-selectivity over various analytes, which is ascribed to the adoption of a boronate moiety. In addition, probe 1 displays an 81 nm red-shift of absorption spectra and the color changes from colorless to yellow upon addition of H2O2, and thus can serve as a “naked-eye” probe for H2O2. Importantly, probe 1 can detect H2O2 quantitatively by ratiometric fluorescence method with a 100 nm red-shifted emission with excellent sensitivity. We highlight the simplicity of the design and synthesis, yet its combined properties, such as high specificity and sensitivity, fast response, visual and ratiometric fluorescent determination with a large red-shifted emission and ratiometric bioimaging in living cells, and anticipate that this probe would be of great benefit to biological researchers for investigating the function of H2O2 in living systems. Our proposed strategy by modulation of the benzyl protecting 4-hydroxynaphthalimide provides a promising methodology for the design of ratiometric fluorescent probe with a large emission shift.Acknowledgements
We gratefully acknowledge financial support from the National Nature Science Foundation of China (no. 21275018, 21107029 and 21203008), Outstanding Young Scientists Award Fund of Shandong Province (BS2013HZ007), Postdoctoral Science Foundation of China (2013M541953), Research Fund for the Doctoral Program of Higher Education of China (RFDP) (no. 20121101110049) and the 111 Project (B07012) for financial support.References
- K. H. Xu, B. Tang, H. Huang, G. W. Yang, Z. Z. Chen, P. Li and L. G. An, Chem. Commun., 2005, 5974–5976 RSC.
- E. W. Miller and C. J. Chang, Curr. Opin. Chem. Biol., 2007, 11, 620–625 CrossRef CAS PubMed.
- L. Yuan, W. Y. Lin, Y. N. Xie, B. Chen and S. S. Zhu, J. Am. Chem. Soc., 2012, 134, 1305–1315 CrossRef CAS PubMed.
- B. Zhu, H. Jiang, B. Guo, C. Shao, H. Wu, B. Du and Q. Wei, Sens. Actuators, B, 2013, 186, 681–686 CrossRef CAS.
- B. C. Dickinson, C. Huynh and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 5906–5915 CrossRef CAS PubMed.
- A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed.
- Z. Q. Ye, J. X. Chen, G. L. Wang and J. L. Yuan, Anal. Chem., 2011, 83, 4163–4169 CrossRef CAS PubMed.
- B. C. Dickinson, Y. Tang, Z. Y. Chang and C. J. Chang, Chem. Biol., 2011, 18, 943–948 CrossRef CAS PubMed.
- W.-K. Oh, Y. S. Jeong, S. Kim and J. Jang, ACS Nano, 2012, 6, 8516–8524 CrossRef CAS PubMed.
- L. P. Du, N. T. Ni, M. Y. Li and B. H. Wang, Tetrahedron Lett., 2010, 51, 1152–1154 CrossRef CAS PubMed.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640–9641 CrossRef CAS PubMed.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- C. Chung, D. Srikun, C. S. Lim, C. J. Chang and B. R. Cho, Chem. Commun., 2011, 47, 9618–9620 RSC.
- G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518–3520 RSC.
- Y. Y. Qian, L. Xue, D. X. Hu, G. P. Li and H. Jiang, Dyes Pigm., 2012, 95, 373–376 CrossRef CAS.
- G. P. Li, D. J. Zhu, Q. Liu, L. Xue and H. Jiang, Org. Lett., 2013, 15, 924–927 CrossRef CAS PubMed.
- W. Lin, L. Long, L. Yuan, Z. Cao, B. Chen and W. Tan, Org. Lett., 2008, 10, 5577–5580 CrossRef CAS PubMed.
- W. Xuan, C. Chen, Y. Cao, W. He, W. Jiang, K. Liu and W. Wang, Chem. Commun., 2012, 48, 7292–7294 RSC.
- M. Kumar, N. Kumar, V. Bhalla, P. R. Sharmab and Y. Qurishib, Chem. Commun., 2012, 48, 4719–4721 RSC.
- B. Zhu, X. Zhang, Y. Li, P. Wang, H. Zhang and X. Zhuang, Chem. Commun., 2010, 46, 5710–5712 RSC.
- B. Zhu, F. Yuan, R. Li, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 7098–7100 RSC.
- B. Zhu, C. Gao, Y. Zhao, C. Liu, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 8656–8658 RSC.
- M. C. Y. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392–15393 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |