Green synthesis of self assembled silver nanowire decorated reduced graphene oxide for efficient nitroarene reduction
Abstract
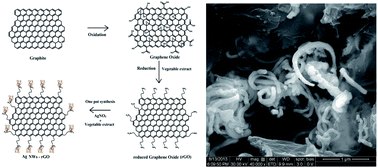
We have developed a facile and green route to prepare reduced graphene oxide (rGO) from graphene oxide (GO) and decorated its surface edges with self assembled Ag Nanowires (Ag NWs). This was carried out by a novel approach using vegetable extract (Abelmoschus esculentus) as a reducing and stabilizing agent. Currently seed mediated and polyol methods are most common in the preparation of any metallic nanowires. These methods would generally be carried out in two steps, whereas in the present study we have developed Ag NWs on a rGO surface (Ag NWs–rGO) in a one step process. Here, the vegetable extract acts as a reductant, functionalizing and capping agent in the reduction of GO followed by the effective functionalization of the rGO surface which in turn in the presence of Ag+ ions, permits the growth of self assembled Ag NWs on the edges of the rGO. The as-prepared Ag NWs–rGO was characterized by UV-visible spectroscopy, XRD, FE-SEM, FT-IR, Raman spectroscopy and elemental analyses. The resultant Ag NWs–rGO exhibits excellent catalytic activity towards the reduction of toxic nitroarene substrates. Importantly, this study could open an avenue for environmentally friendly, simple and cost effective methods in the surface functionalization and generation of self assembled Ag NWs on the edges of rGO nanosheets. Eventually, the unique green reductant used in this work has been proved to be the better reductant and surface functionalizing agent in synthesizing Ag NWs–rGO nanosheets compared to any hazardous chemical reductants.

 Please wait while we load your content...
Please wait while we load your content...