A novel glycopolymeric ultraviolet absorber covering UV-A and UV-B ranges†
Yanfeng Tanga,
Pengfei Gaoa,
Miao Wanga,
Jinli Zhu*a and
Xiangjian Wanb
aSchool of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, PR China. E-mail: jinlizhu@ntu.edu.cn
bSchool of Chemistry, Nankai University, Tianjian, 300071, PR China
First published on 2nd May 2014
Abstract
In this communication, a novel biocompatible polymeric ultraviolet absorber (UVA), poly(HAB-co-OAG-co-AMC) (PHOA), covering UV-A and UV-B ranges was designed and prepared. The photo-antioxidant tests indicate that there is a synergism between UV-A and UV-B monomers. The cytotoxic experiments demonstrate that PHOA has good biocompatibility.
Introduction
The radiation emitted by the sun in the range from 100 to 400 nm is classified into UV-C (100–280 nm), UV-B (280–315 nm) and UV-A (315–400 nm).1 Almost all UV-C radiation, which is harmful to organisms, is absorbed by the higher layers of the atmosphere,2,3 whereas UV-A and UV-B ranges can traverse through the atmospheric layers. It was reported that the excessive UV-B radiation could cause premature skin ageing, sunburns, allergies and even skin cancer.4 However, UV-A radiation is less harmful, and its overdose can also result in similar effects as described above. Therefore, human skin has to be protected against radiation of UV-A and UV-B types. The studies of ultraviolet absorbers (UVAs) have attracted a great deal of attention in recent years. To date, most reported UVAs are small molecules, including benzophenone, cinnamate, hindered amine and triazine.2,5–8In general, these small molecules have excellent UV absorption ability; however, they suffer from several drawbacks, such as being readily absorbed by skin, being incompatible with other composites and poor hydrophilicity. In order to overcome these problems, polymeric UVAs have been developed.9–11 Not only is the shortcoming of being absorbed easily by skin solved due to increased molecular weight, but also incompatibility with other ingredients and poor hydrophilicity are drastically improved by introducing hydrophilic monomers, such as acrylic acid and its derivatives, polyethylene and glycol.
Herein, we aimed to (1) improve the biocompatibility of polymeric UVA, and (2) cover both UV-A and UV-B ranges. As we all know, glucose as a carbohydrate has many advantages, i.e. outstanding biocompatibility, biodegradability and nontoxicity.12–15 Hence, a novel glycopolymeric UVA was designed and prepared through the linkage of UV-A, UV-B and glucosyl monomers by radical copolymerization. The desired polymeric UVA, poly(HAB-co-OAG-co-AMC) (PHOA), was characterized by Fourier transform infrared (FT-IR) spectroscopy and 1H NMR. Its UV absorption performance was investigated by UV-Vis spectroscopy and photo-antioxidant tests. Cytotoxic activity experiments were conducted to evaluate its biocompatibility.
UV-A monomer 2-hydroxy-4-acryloxy benzophenone (HAB) was prepared according to the procedure described in a previous study16 (Scheme 1a), and the UV-B monomer 4-(acryloxy)methyl cinnamate (AMC) was synthesized by two steps (Scheme 1b). PHOA was prepared from HAB, AMC and 3-O-allyl-1,2,5,6-di-O-isopropynylene-α-D-glucose (OAIG) (Scheme 1c).
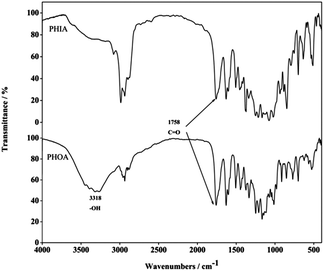
FT-IR spectra of poly(HAB-co-OAIG-co-AMC) (PHIA) and PHOA are shown in Fig. 1. It is obvious that the strong peak at 3318 cm−1 can be assigned to the vibration of OH of the glycosyl group. The presence of this peak reveals that the isopropyl groups protecting the glycosyl ones were removed by hydrolysis and PHOA was successfully prepared from PHIA. The peak at about 1758 cm−1 is assigned to the vibration of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The peaks at 1629 cm−1, 1601 cm−1, 1506 cm−1, and 1445 cm−1 are ascribed to the absorption of aromatic rings, and the absorption band at 1100–1250 cm−1 is the typical vibration of C–O of the ester bond.
O). The peaks at 1629 cm−1, 1601 cm−1, 1506 cm−1, and 1445 cm−1 are ascribed to the absorption of aromatic rings, and the absorption band at 1100–1250 cm−1 is the typical vibration of C–O of the ester bond.
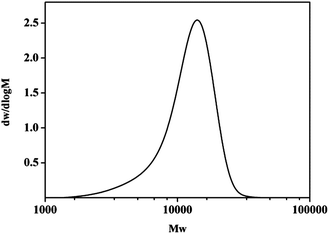
From the GPC spectrum (Fig. 2), it is found that the molecular weight of PHOA is about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542. Only one peak in GPC and FT-IR data shows that the three monomers have copolymerized into PHOA.
542. Only one peak in GPC and FT-IR data shows that the three monomers have copolymerized into PHOA.
The chemical structures of PHIA and PHOA were also characterized by 1H NMR in d6-DMSO (Fig. 3). The spectrum of PHIA exhibits the typical peaks of protected glycosyl groups at 1.31 and 1.40 ppm, which are attributed to the protons of isopropyl groups. The intensities of the peaks at 1.31 and 1.40 ppm decreased in that of PHOA, which further proves that the hydrolysis reaction yielded PHOA. The peaks at 6.36–7.63 ppm are assigned to aromatic rings in HAB and AMC. The peaks at 4.02–5.85 ppm are ascribed to the gylcosyl ring.
The ultraviolet absorption spectra of HAB and 2,4-dihydroxybenzophenone (UV-0) are shown in Fig. 4(a). There are two main peaks in each curve. The peak at 330 nm of HAB is ascribed to the n → π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which is similar to that of UV-0. A blue shift of the π → π* transition occurred (HAB: 269 nm, UV-0: 290 nm) because of the decrease in the electron density of the aromatic ring after OH (in position 4) reacted with acryloyl chloride (AC). Fig. 4(b) shows that there is a blue shift of UV-B type from 318 nm to 280 nm after the reaction of OH (position 4) of p-hydroxy-methyl cinnamate (PHMC) with AC to form an ester bond in AMC. The UV-visible spectrum of PHOA is shown in Fig. 4(c). PHOA shows absorption from 260 nm to 350 nm, covering both the UV-A and UV-B regions. In the PHOA absorption curve, the UV-B absorption intensity is much greater than that of UV-A, indicating that both UV monomers exhibit high absorption of UV-B.
O, which is similar to that of UV-0. A blue shift of the π → π* transition occurred (HAB: 269 nm, UV-0: 290 nm) because of the decrease in the electron density of the aromatic ring after OH (in position 4) reacted with acryloyl chloride (AC). Fig. 4(b) shows that there is a blue shift of UV-B type from 318 nm to 280 nm after the reaction of OH (position 4) of p-hydroxy-methyl cinnamate (PHMC) with AC to form an ester bond in AMC. The UV-visible spectrum of PHOA is shown in Fig. 4(c). PHOA shows absorption from 260 nm to 350 nm, covering both the UV-A and UV-B regions. In the PHOA absorption curve, the UV-B absorption intensity is much greater than that of UV-A, indicating that both UV monomers exhibit high absorption of UV-B.
 | ||
| Fig. 4 The UV-Vis absorption spectra of UV-0, HAB, PHC, PHMC, AMC (7 × 10−5 mol L−1 in ethanol) and PHOA (3 × 10−2 g L−1 in ethanol). | ||
The photo-antioxidant abilities of UV monomers and PHOA are listed in Table 1. The relative oxidation rates (RORs) of HAB and AMC are slightly higher than those of UV-0 and PHMC, which indicates that the photo-antioxidant abilities of both UV monomers decreased after the introduction of the acryloyl group. This group weakened the p–π conjugation between the oxygen atom (position 4) and the phenyl ring, which made electron transition difficult. Consequently, the molar absorption coefficient (ε) is decreased too. As per our expectation, the ROR of PHOA is the lowest among the UV monomers, which demonstrates that there is a synergism between HAB and AMC during the inhibition of photo-oxidation. This may be attributed to the absorption of PHOA covering both UV-A and UV-B ranges.
| UV absorbers | λmax | ROR (%) | ε |
|---|---|---|---|
| UV-0 | 321 | 87.2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| HAB | 329 | 89.2 | 7000 |
| PHMC | 312 | 76.7 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| AMC | 282 | 77.4 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
| PHOA | UV-A 320, UV-B 290 | 70.1 |
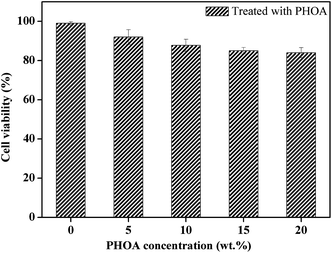
Herein, in order to demonstrate biocompatibility of PHOA, its cytotoxicity was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using well-grown passage L929 mouse cells. The value of cell viability was expressed as a percentage of the control cells, which were cultured with the culture medium only (viability 100%). As shown in Fig. 5, there is only a little decrease in cell viability for all concentrations. This reveals that PHOA has no obvious effect on cell viability.
Conclusions
A novel glycopolymeric UVA, PHOA, was prepared from UV-A, UV-B and glucosyl monomers. PHOA has a strong broad absorption band from 260 to 350 nm, which covers both UV-A and UV-B ranges. The photo-antioxidant data demonstrate that there is a synergism between UV-A and UV-B monomers. These results indicate that PHOA is a promising glycopolymeric UVA having good biocompatibility and its absorption covers both UV-A and UV-B ranges, which makes it a potential candidate as a cosmetics additive.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21376124, 21006054) and Natural Training Programs of Innovation for Undergraduates (no. 201310304019Z).Notes and references
- L. A. Schiave, R. S. Pedroso, R. C. Candido, D. W. Roberts and G. U. Braga, Photochem. Photobiol., 2009, 85, 205–213 CrossRef CAS PubMed.
- K. H. Nquyen, M. C. Kruqler, N. Gouault and S. Tomasi, Nat. Prod. Rep., 2013, 30, 1490–1508 RSC.
- A. Cursino, J. Gardolinski and F. Wypych, J. Colloid Interface Sci., 2010, 347, 49–55 CrossRef CAS PubMed.
- N. Abidi, E. Hequete, S. Tarimala and L. L. Dai, J. Appl. Polym. Sci., 2007, 104, 111–117 CrossRef CAS.
- L. Avar and K. Bechtold, Polym. Degrad. Stab., 1999, 35, 11–17 CAS.
- F. Gugumus, Polym. Degrad. Stab., 2002, 75, 309–320 CrossRef CAS.
- C. M. Seubert, M. E. Nichols, V. A. Cooper and J. L. Gerlock, Polym. Degrad. Stab., 2003, 75, 103–115 CrossRef.
- A. A. Basfar and K. M. I. Ali, Polym. Degrad. Stab., 2006, 291, 437–443 CrossRef PubMed.
- T. Kos, A. Anžlovar, D. Pahovnik, E. Žagar, Z. C. Orel and M. Žigon, Macromolecules, 2013, 46, 6942–6948 CrossRef CAS.
- T. C. Cao, K. L. Xu, G. M. Chen and C. Y. Guo, RSC. Adv., 2013, 3, 6282–6285 RSC.
- D. R. Kelsey, B. M. Scardino, J. S. Grebowicz and H. H. Chuah, Macromolecules, 2000, 33, 5810–5818 CrossRef CAS.
- X. P. Chen and N. Ayres, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3030–3037 CrossRef CAS.
- Y. S. Huang, L. Taylor, X. P. Chen and N. Ayres, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 5230–5238 CrossRef CAS.
- X. P. Chen and N. Ayres, Macromolecules, 2010, 43, 1341–1348 CrossRef CAS.
- L. Taylor, X. P. Chen and N. Ayres, Polym. Int., 2014, 63, 127–135 CrossRef CAS.
- S. Tragoonwichian, A. E. O’Rear and N. Yanumet, Colloids Surf., A, 2008, 329, 87–94 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental operation, images and additional spectra. See DOI: 10.1039/c4ra01768d |
| This journal is © The Royal Society of Chemistry 2014 |