A new fluorescent probe based on styrylcyanine dye containing pyridine: dissimilar fluorescent response to Cu2+ and Pb2+†
Xiaodong Yangb,
Weifeng Zengb,
Lei Wangb,
Xinwei Lua,
Yichen Yana,
Jinqing Qu*b and
Ruiyuan Liu*a
aSchool of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, P.R. China. E-mail: ruiyliu@smu.edu.cn; Fax: +8602061648196; Tel: +8602061648196
bSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, P.R. China. E-mail: cejqqu@scut.edu.cn
First published on 13th May 2014
Abstract
A highly sensitive fluorescent probe (1) based on styrylcyanine dye for Cu2+ and Pb2+ has been developed. Probe 1 exhibited fluorescent turn-off sensing ability to Cu2+. On the other hand, 1 displayed ratiometric fluorescent response towards Pb2+ with a distinct fluorescent color change from blue to orange. The 1H NMR titrations revealed that the fluorescent response of 1 to Cu2+ and Pb2+ is triggered by the interaction of the pyridine unit and the metal ion. Detection limits of 1 to Cu2+ or Pb2+ were calculated as 1.24 and 3.41 × 10−6 M, respectively, by standard deviation and linear fitting. Furthermore, 1 can also be used as a sensor for detection of Cu2+ and Pb2+ in a test strip.
Introduction
The design and synthesis of fluorescent probes that allow selective and sensitive detection of toxic heavy and transition metal ions have attracted considerable attention.1 Fluorescent techniques offer the feasibility of fast, facile and highly sensitive detection of target analyte.2 On the other hand, these metal ions have caused health and environmental problems.3 Especially, Cu2+ and Pb2+ have been regarded as the toxic metal ions. For example, Cu2+ is an important trace element which plays crucial roles in various biological processes.4 In addition to these crucial roles, Cu2+ is a significant environmental pollutant and has toxic effect on organisms especially at high concentration levels, since it can displace other metal ion that act as cofactor in enzyme-catalyzed reaction, thus causing Alzheimer's, Parkinson's and Wilson diseases, hypoglycemia, dyslexia and infant liver damage.5 Pb2+ is one of the most toxic heavy metal ions. Intake of even very small amount of Pb2+ causes several health problems such as memory loss, anemia, and slow nerve conduction velocity in children.6Hence, there are many efforts devoted to the development of fluorescent sensors for Cu2+ and Pb2+. A variety of fluorescent Cu2+ or Pb2+sensors based on either metal-induced chemical reaction or coordination have been synthesized.7 For instance, Zhao et al. reported fluorescent Cu2+ sensor based on copper metal-induced opening of spirolactam ring in the xanthenes.8 Xu and colleagues synthesized fluorescent Cu2+ sensor based on the chelation of Cu2+ with chemosensor.9 Yoon and co-workers designed a fluorescent sensor based on rhodamine B for the detection of Pb2+.10 Huang et al. developed a fluorescent probe based on calix[4]arene derivative which exhibited highly selective fluorescent response to Pb2+ over alkali, alkali earth metal ion and some transition metal ion.11
Owing to the importance of Cu2+ and Pb2+, many probes are separately available as mentioned previously, but sensor with dissimilar response to Cu2+ and Pb2+ is cost-effective and highly desirable for real time application. However, developments of such sensor are challenging tasks and also have the synthetic difficulties.
Recently, nitrogen heterocyclic derivatives such as pyridine, bipyridine, pyrrole, and quinoline have been used as receptor for fluorescent sensor due to their binding affinities to metal ion.12 The chemosensors containing these nitrogen heterocyclic compounds exhibited sensitive response to heavy metal ions, but only a few of them were accounted for by multiple analyte recognition.13 Herein, in this work, we reported a styrylcyanine dye containing pyridine unit as fluorescence sensor for highly selective detection of Cu2+ and Pb2+ (Scheme 1).
Results and discussion
Compound 1 was synthesized from the reaction of 1,1,2-trimethylbenz[e]indole and 4-pyridinecarboxaldehyde, and characterized by NMR, IR, and ESI-MS (Fig. S1–S4†).We first evaluated the capability of 1 to detect copper ion in solution. The titration of Cu2+ to 1 was performed in CH3CN–water mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 1a). The free probe displays a blue emission with the maximum at 475 nm; upon addition of Cu2+, the fluorescence intensity of 1 in the emission spectra decreases. The addition of 120 μM Cu2+ quenches 14% of the original fluorescence.
1, v/v) (Fig. 1a). The free probe displays a blue emission with the maximum at 475 nm; upon addition of Cu2+, the fluorescence intensity of 1 in the emission spectra decreases. The addition of 120 μM Cu2+ quenches 14% of the original fluorescence.
As shown in Fig. 1b, a linear relationship is observed between the fluorescent intensity and the Cu2+ amount at concentrations lower than 90 μM. The Stern–Volmer quenching constant (kSV = 8.05 × 106 M−1, R = 0.995), which is calculated via a linear analysis of the fluorescence intensity versus the Cu2+ ion concentration, suggests a strong binding ability of 1 towards Cu2+. The detection limit for Cu2+ is determined as 1.24 μM based on S/N = 3, which is sufficiently low to allow the fluorescent detection of micromolar concentration of Cu2+.
To gain insight into the luminescent response of 1, 1H NMR titrations of 1 in the presence of Cu2+ were performed (Fig. 2). All protons were assigned referring to the literature. The pattern of 1H NMR titration indicates that the Cu2+ coordination triggers a downfield shift and disappearance of aromatic proton Ha of the pyridine moiety. It suggests that the pyridine unit participates in binding to Cu2+, which decreases proton exchange, reduces π-electron density, improves the PET or energy transfer (ET) process and results in a considerable quenching in fluorescence. Moreover, a slight down-field shift and disappearance of a broad signal of Ha proton indicates the involvement N in bonding to the Cu2+. The broadening of 1H NMR signals ascribes to the usual paramagnetic effect of Cu2+.
 | ||
| Fig. 2 Partial 1H NMR spectra of 1 upon addition of Cu2+ in DMSO-d6. (a) [Cu2+]/[1] = 0, (b) [Cu2+]/[1] = 0.5, (c) [Cu2+]/[1] = 1. | ||
The fluorescent response of 1 to Pb2+ was also evaluated (Fig. 3). Upon addition of Pb2+, the emission intensity at 475 nm gradually decreases with the simultaneous appearance of a new orange emission peak at 595 nm, and an iso-emission point at 550 nm is observed. All the responses are almost instant. Noteworthy is that the difference between the two emission wavelengths is very large (Δλem = 120 nm), which not only contributes to the accurate measurement of the intensities of the two emission peaks, but also results in a huge ratiometric value. In the presence of Pb2+ of 300 μM, a ca. 16-fold enhancement in the ratiometric value of I595/I475 (from 0.09 to 1.48) is achieved with respect to the Pb2+ free solution. Essentially, the ratio of the emission intensities (I595/I475) becomes constant when the amount of Pb2+ reaches 20 equiv. The dissociation constant Kd of the Pb2+–1 complex was estimated to be 5.82 × 104 M−1 (Fig. 3b). In addition, the detection limit is determined to be 3.41 μM based on S/N = 3.
 | ||
Fig. 3 (a) Change in fluorescence (λex = 350 nm) of 1 (30 μM) in CH3CN–water mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) upon addition of Pb2+. (b) Plot of I595/I475 versus concentration of Pb2+. 1, v/v) upon addition of Pb2+. (b) Plot of I595/I475 versus concentration of Pb2+. | ||
1H NMR titration experiments were also performed to explore the coordination mechanism of 1 and Pb2+ (Fig. S5†). The complex process of 1 in DMSO-d6 induces noticeable downfield shift of the pyridine ring proton. It seems that the N atom in pyridine ring is also involved directly binding to the Pb2+, which is in accordance with the phenomena reported in the previous paper.10
The selectivity of 1 towards Cu2+ or Pb2+ over relevant metal ions was then evaluated by measuring the optical changes upon addition of other metal ions (Fig. 4 and S6†). The fluorescent spectra of 1 exhibits only slightly change upon addition of representative metal ions such as K+, Na+, Ca2+, Ag+, Mg2+, Hg2+, Co2+, Fe2+, Mn2+, Pd2+, Cd2+, Ni2+, Zn2+. When Cu2+ is added, the fluorescence of 1 is quenched. We further examined the fluorescence response of 1 toward Cu2+ in the presence of other potentially competing meal ions (Fig. S7 and S8†). The titration of Cu2+ and 1 in the presence of various metal ions was conducted, and the experimental results indicate that most of the relevant metal ions only display minimum interference. These results indicate the excellent selectivity of 1 towards Cu2+ over the other competitive metal ions.
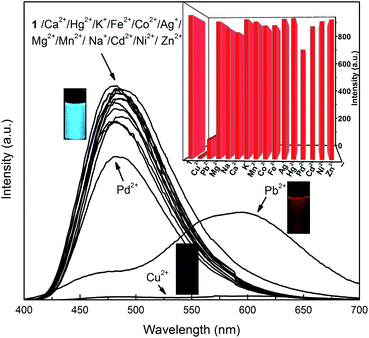 | ||
Fig. 4 Fluorescent spectra of 1 (30 μM) in presence of metal ions in CH3CN–water mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) ([1] = 30 μM, [metal ion] = 150 μM). 1, v/v) ([1] = 30 μM, [metal ion] = 150 μM). | ||
On the other hand, 1 displays a selective fluorescent behaviour towards Pb2+ with a fluorescence change from blue to orange, which can be easily seen by the naked eyes (Fig. 5). Under the same conditions, nearly no ratiometric response changes are observed in the presence of other metal ions.
 | ||
Fig. 5 Photograph of 1 in the presence of a series of metal ions in CH3CN–water mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) ([1] = 1.0 mM, [metal ion]/[1] = 20). Irradiation under 365 nm. 1, v/v) ([1] = 1.0 mM, [metal ion]/[1] = 20). Irradiation under 365 nm. | ||
In addition to the “wet” measurements, we wonder whether 1 could also work in test strip. For real-world application, it is preferable to perform the detection on test paper because this requires no complex and expensive equipment and is thus simple, quick and convenient. First, we prepared a paper film containing 1 by immersing a filter paper into the THF solution of 1 (1.0 mM) and then drying it in the air. As displayed in Fig. 6a, the dye-loaded paper emits bright green light upon photo excitation. Addition of the aqueous copper ion containing solution, the color of the paper films change from pale yellow to yellow, and the fluorescence was quenched. Interesting, the fluorescent color of dye-loaded paper films change from green to yellow, after immersed into aqueous lead ion containing solution. To confirm the influence of other metal ions on the Cu2+ or Pb2+ detection, test papers were immersed in corresponding aqueous solutions in the presence of K+. Similar emission color changes are observed. This demonstrates a prototype device using 1 for detecting copper ion and lead ion in the wilderness.
 | ||
| Fig. 6 (a) The fluorescent changes and (b) color changes of the test papers for detecting Cu2+ or Pb2+. ([Cu2+] = 3.9 mg L−1, [Pb2+] = 24.6 mg L−1). | ||
Conclusions
In conclusion, we have designed and synthesized a styrylcyanine dye containing pyridine unit as fluorescence sensor for detection of Cu2+ and Pb2+. Probe 1 exhibits fluorescence turn-off response to Cu2+, but 1 shows ratiometric fluorescent sensing to Pb2+. 1H NMR titrations of 1 reveals that the fluorescent response of 1 is triggered by the interactions of pyridine unit and Cu2+/Pb2+. The typical detection limits (DL) of 1 towards Cu2+ and Pb2+ are calculated as 1.24 and 3.41 μM, respectively, by standard deviation and linear fitting. Furthermore, 1 can also be used as a chemosensor for detection of Cu2+ and Pb2+ in test strip.Experimental section
Materials
All chemicals and solvents were of analytical grade and were used without further purifications. 1,1,2-Trimethyl-1H-benzo[e]indole and 4-pyridinecarboxaldehyde were purchased from Sigma-Aldrich company. All other chemicals were commercially available from J&K Scientific Ltd.The stock solutions of metal ions for selectivity experiments were prepared respectively by dissolving KCl, NaCl, CaCl2, Cu(NO3)2·2H2O, Pb(NO3)2, Zn(NO3)2, HgCl2, Ni(NO3)2·6H2O, Cd(NO3)2·4H2O, MnCl2·4H2O, AgNO3, CoCl2·6H2O, and FeCl2·4H2O in doubly distilled water.
Instruments
1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded on a Bruker Avance/DMX 400 MHz NMR spectrometer with DMSO-d6 as solvent and tetramethylsilane as an internal reference. IR spectra were measured using a Shimadzu FTIR-8100 spectrophotometer. Melting points (mp) was measured on a Yanaco micro-melting point apparatus. Mass spectra (MS) were carried out on a GCT Premier CAB 048 mass spectrometer operating in a chemical ionization mode (CI). Elemental analysis was performed on an Eager 300 elemental microanalyzer. UV-vis spectra were recorded in a quartz cell (thickness: 1 cm) at room temperature using a JASCO J-820 spectropolarimeter. Fluorescence spectra were measured on FLS-920 Edinburgh Fluorescence Spectrophotometer, with a xenon lamp and 1.0 cm quartz cells.Synthesis of 1
A mixture of 2.09 g (10.00 mmol) 1,1,2-trimethyl-1H-benzo[e]indole and 1.07 g (10.00 mmol) 4-pyridinecarboxaldehyde were refluxed in 50 mL anhydride ethanol with one drop piperidine for 24 h. After the solvent was removed under reduced pressure, the yellow solid was purified by column chromatography using methylene chloride as the eluent. 1.91 g of yellow product was obtained. Yield: 64%. 1H NMR (400 MHz, DMSO-d6) δ (ppm) = 8.65 (d, J = 4.7, 2H), 8.19 (d, J = 8.4, 1H), 8.04 (d, J = 8.2, 1H), 7.97 (d, J = 8.5, 1H), 7.82 (d, J = 13.2, 7.7, 4H), 7.69 (s, 1H), 7.67–7.60 (m, 1H), 7.52 (t, J = 7.5, 1H), 1.65 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ = 184.29, 150.74, 150.20, 143.00, 139.83, 134.40, 132.20, 129.46, 129.05, 128.04, 126.75, 124.89, 123.59, 123.05, 121.72, 120.18, 54.22, 21.85. IR (v−1, LiBr): 3050, 2973, 2927, 2836, 1595, 1545, 1502, 1461, 1415, 1217, 970, 823, 744. MS (ESI): C21H18N2 m/z 298.1470 for [M] + H+ 299.1544. Elemental analysis: calcd C, 84.53; H, 6.08; N, 9.39. Found C, 85.04; H, 6.11; N, 8.85. mp: 246.5–247.0 °C.Absorption and fluorescence analysis
A typical experimental procedure is described as follows: stock solutions of 1 (CH3CN, 3 mM) and Cu(NO3)2·2H2O (DI water, 30 mM) were prepared in flask equipped with stopcock, respectively. 1 solution (0.1 mL) and the Cu(NO3)2 solution (0.1 mL) were transferred to a vial, and then the resulting mixture was diluted to 10 mL with CH3CN–water mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to give the sample solution, of which [1] and [Cu2+] were adjusted to be 30 μM and 300 μM, respectively. The concentration of 1 was 30 μM throughout the analysis experiments except that otherwise pointed out. The fluorescence intensity was measured with the excitation wavelength 350 nm except as otherwise noted, and the excitation and emission slits were set to 1 and 1 nm, respectively.
1, v/v) to give the sample solution, of which [1] and [Cu2+] were adjusted to be 30 μM and 300 μM, respectively. The concentration of 1 was 30 μM throughout the analysis experiments except that otherwise pointed out. The fluorescence intensity was measured with the excitation wavelength 350 nm except as otherwise noted, and the excitation and emission slits were set to 1 and 1 nm, respectively.
Acknowledgements
We gratefully thank the “National Natural Science Foundation of China” (51173050 and 21244007) and “Medical Scientific Research Foundation of Guangdong Province, China” (A2010355) for financial support of this work.Notes and references
- (a) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed; (b) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed; (c) M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410–2433 RSC; (d) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- (a) S. E. Angell, C. W. Rogers, Y. Zhang, M. O. Wolf and W. E. Jones, Chem. Rev., 2006, 250, 1829–1841 CAS; (b) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS PubMed.
- (a) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190–1193 CrossRef CAS PubMed; (b) G. Zhang, B. Y. Lu, Y. P. Wen, L. M. Lu and J. K. Xu, Sens. Actuators, B, 2012, 171–172, 786–794 CrossRef CAS PubMed; (c) J. S. Kim and D. T. Quang, Chem. Rev., 2010, 110, 6280–6301 CrossRef PubMed; (d) T. T. Zhang, F. W. Wang, M. M. Li, J. T. Liu, J. Y. Miao and B. X. Zhao, Sens. Actuators, B, 2013, 86, 755–760 CrossRef PubMed.
- (a) H. Ye, F. Ge, X. C. Chen, Y. Li, H. Zhang, B. X. Zhao and J. Y. Miao, Sens. Actuators, B, 2013, 182, 273–279 CrossRef CAS PubMed; (b) I. A. Koval, P. Gamez, C. Belle, K. Selmeczi and J. J. Reedijk, Chem. Soc. Rev., 2006, 35, 814–840 RSC; (c) H. S. Jung, J. H. Han, Z. H. Kim, C. Kang and J. S. Kim, Org. Lett., 2011, 13, 5056–5059 CrossRef CAS PubMed; (d) P. X. Xia, J. Y. Dou, L. Huang, M. Xu, F. J. Chen, Y. J. Wu, D. C. Bai, W. G. Li and Z. Z. Zeng, Sens. Actuators, B, 2010, 148, 337–341 CrossRef PubMed.
- (a) E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed; (b) S. C. Luza and H. C. Speisky, Am. J. Clin. Nutr., 1996, 63, 8125–8205 Search PubMed; (c) S. R. Uauy, M. Olivares and M. Gonzalez, Am. J. Clin. Nutr., 1998, 67, 9525–9595 Search PubMed.
- (a) C. Yang, L. Liu, T. Zeng, D. W. Yang, Z. Y. Yao, Y. L. Zhao and H. C. Wu, Anal. Chem., 2013, 85, 7302–7307 CrossRef CAS PubMed; (b) A. Thakur, D. Mandal and S. Ghosh, Anal. Chem., 2013, 85, 1665–1674 CrossRef CAS PubMed; (c) F. Zapata, A. Caballero, A. Espinosa, A. Tarraga and P. Molina, Org. Lett., 2008, 10, 41–44 CrossRef CAS PubMed; (d) X. Miao, L. Ling and X. Shuai, Chem. Commun., 2011, 47, 4192–4194 RSC.
- (a) P. S. Song, Y. Xiang, H. Xing, Z. J. Zhou, A. J. Tong and Y. Lu, Anal. Chem., 2012, 84, 2916–2922 CrossRef CAS PubMed; (b) K. Kavallieratos, J. M. Rosenberg, W. Z. Chen and T. Ren, J. Am. Chem. Soc., 2005, 127, 6514–6515 CrossRef CAS PubMed; (c) J. Y. Lee, S. K. Kim, J. H. Jung and J. S. Kim, J. Org. Chem., 2005, 70, 1463–1466 CrossRef CAS PubMed; (d) L. Xu, Y. F. Xu, W. P. Zhu, X. L. Sun, Z. Xu and X. H. Qian, RSC Adv., 2012, 2, 6323–6328 RSC.
- (a) W. Y. Liu, H. Y. Li, B. X. Zhao and J. Y. Miao, Org. Biomol. Chem., 2011, 9, 4802–4805 RSC; (b) W. Y. Liu, H. Y. Li, B. X. Zhao and J. Y. Miao, Analyst, 2012, 137, 3466–3469 RSC.
- (a) Z. C. Xu, J. Y. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 2563–2565 RSC; (b) Z. C. Xu, S. J. Han, C. Lee, J. Y. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 1679–1681 RSC.
- J. Y. Kwon, Y. J. Jang, Y. J. Lee, K. M. Kim, M. S. Seo, W. Nam and J. Yoon, J. Am. Chem. Soc., 2005, 127, 10107–10111 CrossRef CAS PubMed.
- J. M. Liu, J. H. Bu, Q. Y. Zheng, C. F. Chen and Z. T. Huang, Tetrahedron Lett., 2006, 47, 1905–1908 CrossRef CAS PubMed.
- (a) J. Han and K. Burgess, Chem. Rev., 2010, 110, 2709–2728 CrossRef CAS PubMed; (b) T. Jokic, S. M. Borisov, R. Saf, D. A. Nielsen, M. Kuhl and I. Klimant, Anal. Chem., 2012, 84, 6723–6730 CrossRef CAS PubMed; (c) T. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 3401–3409 CrossRef CAS PubMed.
- (a) L. Xue, Q. Liu and H. Jiang, Org. Lett., 2009, 11, 3454–3457 CrossRef CAS PubMed; (b) N. Kumari, N. Dey, S. Jha and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 2438–2445 CrossRef CAS PubMed; (c) S. Khatua, S. H. Choi, J. Lee, J. O. Huh, Y. Do and D. G. Churchil, Inorg. Chem., 2009, 48, 179–1801 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02738h |
| This journal is © The Royal Society of Chemistry 2014 |


