Nitrogen/sulfur co-doped non-noble metal material as an efficient electrocatalyst for the oxygen reduction reaction in alkaline media
Li
Xu
*ab,
Guoshun
Pan
*ab and
Xiaolu
Liang
ab
aState Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China. E-mail: xl0522@126.com; pangs@tsinghua.edu.cn; Fax: +86 0755 2695 7008; Tel: +86-755-2671 1824
bShenzhen Key Laboratory of Micro/nano Manufacturing, Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, China
First published on 14th April 2014
Abstract
This work demonstrates the feasibility of nitrogen/sulfur co-doped non-noble metal materials (Fe–N/C–TsOH) as platinum-free catalysts for the oxygen reduction reaction (ORR) in alkaline media. Electrochemical techniques such as cyclic voltammetry (CV), rotating disk electrodes (RDEs) and rotating ring-disk electrodes (RRDEs) are employed with the Koutecky–Levich theory to investigate the ORR kinetic constants and the reaction mechanism. It is found that the catalysts doped with TsOH (p-toluenesulfonic acid) show significantly improved ORR activity relative to a TsOH-free catalyst. The overall electron transfer numbers for the catalyzed ORR are determined to be 3.899 and 3.098, respectively, for the catalysts with and without TsOH-doping. Catalysts heat treated at 600 °C exhibit relatively higher activity. In addition, the catalyst doped with TsOH (Fe–N/C–TsOH-600) not only exhibits exceptional stability in 0.1 mol L−1 KOH solution but also has higher methanol tolerance compared to commercial Pt/C catalyst in 0.1 mol L−1 KOH. To some extent, increasing the Fe–N/C–TsOH-600 loading on the electrode favors a faster reduction of H2O2 to intermediate to H2O. X-ray photoelectron spectroscopy analysis indicates that pyrrolic N groups are the most active sites, and that sulfur species are structurally bound to carbon in the forms of C–S(n)–C and oxidized –SO(n)– bonds, an additional beneficial factor for the ORR.
1. Introduction
Polymer electrolyte membrane (PEM) fuel cells are considered a promising technology to replace internal combustion engines for automotive propulsion, due to their high energy yield and low environmental impact. Notable among these are direct methanol alkaline fuel cells (DMAFCs), which have attracted considerable interest as alternative power sources for automobiles and portable consumer electronics.1 Since alcohols have a similar distribution infrastructure to petroleum, they can be easily handled, stored, and transported, and they promise major improvements over gasoline combustion, including better overall fuel efficiency and lower emissions.2 Nevertheless, the sluggish kinetics of the cathodic ORR is a major factor hampering large-scale implementation of DMAFCs, so most research in this area currently focuses on developing efficient catalysts for the ORR.3The ORR process can occur along a 4-electron pathway to directly produce H2O or a less efficient 2-electron pathway involving the formation of H2O2 as an intermediate.4,5 Although platinum-based nanomaterials are the most efficient ORR catalysts, they still face multiple problems, such as unstable catalytic activity, poor stability, limited supply, and high cost.5–7 As well, due to anode methanol penetration, oxygen reduction and methanol oxidation occur simultaneously on the Pt surface, forming “cathodic mixed potential” and decreasing the cell performance as well as poisoning the cathode catalyst's Pt.8–10 Thus, developing cost-effective catalysts with high ORR catalytic activity, long-term stability, and methanol tolerance is crucial for advancing DMAFCs.
Owing to the inherently faster electrode kinetics and less corrosive conditions in alkaline media, a large number of catalysts have been investigated for the ORR in DMAFCs. Catalysts synthesized by pyrolyzing precursors comprising nitrogen, carbon, and transition metals have drawn significant attention because of their reasonable activity levels, remarkable selectivity in the catalysis of the ORR, and high tolerance to methanol, even though the mechanisms of the catalyst reaction during the heat-treatment process and the resulting improvement in activity are complicated and not fully understood.2,11–13 Chu and Jiang14,15 prepared a series of single and binary heat-treated metalloporphyrins (TPP as a ligand), and tested the ORR in O2-saturated 0.5 mol L−1 H2SO4 with 1 mol L−1 methanol. The best catalyst for oxygen reduction, which was also inert to methanol oxidation, was heat-treated Co–/Fe–TPP. Chu and Jiang proposed that the macrocycles were only partially decomposed by the heat treatment (600 °C in Ar), the carbonized organic groups could form a nanostructure and the catalytic sites were MN4 core. Holze et al. investigated carbon-supported Fe–TMPP (TMPP stands for tetramethoxyphenylporphyrin) heated treated at 800 °C as an active catalyst for the ORR in fuel cells using methanol as the fuel and an alkaline carbonate electrolyte.16 The pyrolyzed Fe–TMPP catalyst was as active as Pt/C and less sensitive toward the establishment of a mixed potential. Indeed, upon the addition of 1 mol L−1 methanol, the polarization curve of the chelate-load electrode remained unchanged, whereas the Pt/C electrode showed much poorer performance.
Very recently, carbons doped or co-doped with other heteroatoms, such as B,17 P,18,19 and S,20,21 have been developed and have shown good catalytic activity for the ORR by taking advantage of the different heteroatoms in the conjugated carbon backbone that can create new non-electroneutral sites. For instance, S and N co-doped carbons have shown better tolerance to methanol than commercial Pt/C electrode.22 Nevertheless, some parameters, such as onset potential, half-wave potential, and current density, still need to be improved.
In this work, TsOH and polypyrrole (PPy) were used as dual dopants to synthesize a novel non-precious metal catalyst, Fe–N/C–TsOH. TsOH, an organic compound, is known as a tosyl group and was used not only as the S precursor but also as an “organic-soluble” acid catalyst to promote the oxidation of pyrrole. ORR activity and methanol tolerance were assessed using CV, RDE and RRDE techniques. Powder X-ray diffraction (XRD) as well as X-ray photoelectron spectroscopy (XPS) were employed to confirm the material properties in terms of morphology and chemical speciation. We demonstrated that the catalyst doped with TsOH showed much better electrocatalytic activity for ORR performance than a catalyst without TsOH doping. We then focused on evaluating Fe–N/C–TsOH catalyst heat treated at 600 °C. In particular, the effect of KOH concentration on its ORR catalytic properties was examined using the RRDE technique in 0.1–5.0 mol L−1 KOH electrolyte. The effect of heat treatment on the catalyst's methanol tolerance was studied, and we compared this tolerance with that of commercially available Pt/C to evaluate the treated catalyst's applicability in DMAFCs.
2. Experimental
2.1. Preparation and physical characterization of the catalysts
To prepare the nitrogen and sulfur dual-doped non-precious metal electrocatalyst (Fe–N/C–TsOH), we mixed 50 mg pyrrole (chemically pure) and 10 mL methanol solvent (analytically pure; both purchased from Guoyao, Shanghai), then combined this mixture with 120 mg carbon black (Vulcan XC 72R with a BET of 235 m2 g−1, purchased from Cabot), followed by 10 min of ultrasonication. 0.25 mL 30% H2O2 and 50 mg TsOH (analytically pure, purchased from Guoyao) were added to this ultrasonicated suspension while being ground in a mortar for some time to obtain a slurry. 10 mL methanol with 149 mg FeSO4·7H2O (analytically pure) was then added to the mortar, followed by constant milling for another 45 min, then drying in a vacuum at 60 °C for 1 h to obtain a powder. This powder was further processed by thermal treatment under N2 atmosphere at temperatures of 200, 400, 600, and 700 °C, respectively, for 2 hours to optimize the pyrolysis temperature with respect to ORR electrocatalytic activity. To elucidate the effect of TsOH alone, a baseline sample of carbon loaded with TsOH-free Fe–N was also prepared under the same conditions described above and is denoted as Fe–N/C. Furthermore, the Fe–N/C–TsOH-600 catalyst was leached in 0.5 mol L−1 H2SO4 at 80 to 90 °C for some hours to remove excess metals on the surface of the catalyst (denoted as Fe–N/C–TsOH-600L). Then the resulting samples were filtered, washed, and subjected to a second heat treatment at 600 °C under N2 for 2 h (denoted as Fe–N/C–TsOH-600LH). In the relevant figure's legend, “RT” means untreated or unpyrolyzed catalyst and “600” indicates the sample pyrolyzed at 600 °C.Structural and crystal-phase analyses of the catalysts were performed using XRD with Cu Kα radiation (λ = 0.15406 nm), operating at 40 kV and 40 mA. Surface analysis of the catalysts was conducted by XPS on a PHI Quantera Scanning X-ray Microprobe™ 5300 system (ULVAC-PHI. INC) with an Al K X-ray anode source (hν = 1486.6 eV) at 300 W and 15.0 kV.
2.2. Electrochemical measurements
Electrochemical measurements were performed in a conventional three-electrode cell using the RRDE technique in KOH solution, ranging in concentration from 0.1 to 5.0 mol L−1, at room temperature. A rotating glassy carbon disk electrode (glassy carbon electrode with a geometric surface area of 0.19625 cm2, purchased from Gamry Instruments) was coated with catalyst to form the catalyst layer and functioned as the working electrode. According to the electrode preparation method described by Qiao et al.,2 aliquots of 5, 10, 20, 30, and 40 μL of homogeneous catalyst ink, consisting of 2.0 mg catalyst mL−1, were pipetted onto the glassy carbon (GC) disk electrode. The catalyst loadings were 50.9, 101, 203.6, 305.4, and 407.2 μm g cm−2. A saturated calomel electrode (SCE) and Pt wire were used as the reference and counter electrodes, respectively. All measured potentials were converted to the standard hydrogen electrode (SHE).During the measurement, CVs were first carried out on the freshly prepared working electrode by repeatedly cycling the potential between 0.80 and 0.30 V for more than 30 cycles at a scan rate of 50 mV s−1 in the presence of N2 bubbling. In this case, the electrode surface was determined to be clean when a stable, reproducible voltammogram was recorded. The ORR activity of the catalyst was tested in O2-saturated KOH solutions. For more quantitative measurements of the ORR activity, linear sweep voltammetry (LSV) was conducted on the catalyst-coated RDE in the potential range between 0.76 and 0.15 V at a scan rate of 5 mV s−1 in O2-saturated KOH solution at various rotation rates, from 300 to 2100 rpm.
3. Results and discussion
3.1. Electrochemical activity of Fe–N/C–TsOH catalysts towards the ORR
LSV measurements of the catalysts prepared without and with TsOH in O2-saturated 0.1 mol L−1 KOH solutions were taken at 5 mV s−1, and the results are presented in Fig. 1. Both Fe–N/C–TsOH-600 and Fe–N/C-600 are catalytically active for the ORR. However, Fe–N/C–TsOH-600 gives much higher ORR activity than Fe–N/C-600 if both their onset potentials (Eonset) and their half-wave potentials (E1/2) are compared. In addition, the maximum current density for Fe–N/C-TsOH-600 is 1.7 times higher than that of Fe–N/C-600. In other words, the addition of TsOH considerably enhances the former catalyst's activity relative to the latter's. According to the literature, the dopant breaks the electroneutrality of the carbon material, since the difference in electroneutrality between carbon and dopant creates favorable positively charged sites for side-on O2 surface adsorption.17 As well, pyrolysis of the catalyst in the presence of sulfur leads to mainly amorphous carbon, resulting in increased catalyst porosity and, in turn, enhanced catalyst performance.23,24 Furthermore, sulfur (or a sulfo group), whose electronegativity is close to that of carbon, has been employed as a dopant in the preparation of M–Nx catalysts, and the sulfur group doped to M–Nx–C might be helpful for entrapping M ions in an environment rich in pyrrole-type or pyridine-type nitrogen.20The difference between the diffusion-limiting currents of Fe–N/C–TsOH-600 and Fe–N/C-600 may suggest that the ORR mechanisms they catalyze are different, particularly in terms of the overall electron transfer number. The inset graph in Fig. 1 shows CVs of the two catalysts, which reinforce the LSV results. A more positive peak potential value and a higher peak current density were obtained than with Fe–N/C-600, indicating better ORR activity after TsOH-doping. We therefore believe that the TsOH or the sulfur plays a key role in improving the electrocatalytic performance of these carbon materials for the ORR.
For a more quantitative evaluation of the ORR catalytic activity of the dual-doped catalysts developed in this work, further RDE voltammetry measurements were carried out at different electrode rotation rates, from 300 to 2100 rpm, as shown in Fig. 2(a) and (b). The overall electron transfer number (n) during the ORR process can be evaluated from the Koutecky–Levich equation:2
| id−1 = ik−1 + (0.201nFCO2DO22/3ν−1/6ω1/2)−1 | (1) |
The average n values were determined to be 3.098 for Fe–N/C-600 and 3.899 for Fe–N/C–TsOH-600, the latter being very close to 4. Clearly, the catalysts doped with TsOH show a much higher overall ORR electron number than those without TsOH doping, indicating a significant difference in the mechanisms they catalyze. In other words, the ORR catalyzed by Fe–N/C–TsOH-600 is a 4-electron transfer process from O2 to H2O, whereas Fe–N/C-600 generally tends to catalyze the ORR through a (2 + 2)-electron pathway, producing H2O2, which is capable of oxidizing and splitting active sites.
The intercepts for all the Koutecky–Levich plots are slightly higher than 0. A non-zero intercept for a Koutecky–Levich plot demonstrates that the reaction can occur within the pores of the catalyst rather than just on the top surface exposed to the bulk solution, thus increasing the active surface area at slower kinetics and thereby affecting the slope.25,26 Therefore, it can be concluded that the ORR catalyzed by Fe–N/C–TsOH-600 is much faster than that catalyzed by Fe–N/C-600.
As the potential increases, the Koutecky–Levich slope increases for both catalysts, suggesting a decrease in the number of electrons transferred during the ORR and, thus, a reduction in H2O selectivity. In addition, the intercepts of the Koutecky–Levich plots become much greater than 0, suggesting a decrease in the kinetics of the catalyst for the ORR. However, the Koutecky–Levich slopes at different electrode potentials for Fe–N/C–TsOH-600 all are much quicker than those for Fe–N/C-600. This further demonstrates that the Fe–N/C has a large active surface area and uniform dispersion of the catalyst on the electrode surface after TsOH doping, hence the faster kinetics for the ORR catalyzed by Fe–N/C–TsOH-600 compared to Fe–N/C-600.
Furthermore, we measured the polarization curves using the RDE technique in O2-saturated 0.1 mol L−1 KOH solution for catalysts pyrolyzed from 200 to 700 °C, with the unpyrolyzed catalyst sample (Fe–N/C–TsOH) for comparison, to clarify the effect of thermal treatment on the ORR activity. The results are presented in Fig. 3. Evidently, the pyrolyzed catalyst samples all show better ORR activities than the unpyrolyzed sample, which is consistent with the common belief that thermal treatment can effectively improve catalysts' ORR activity.22,27,28 With increasing pyrolysis temperature, the ORR activity keeps rising up to 600 °C, then starts to decrease at higher temperatures. This suggests that 600 °C may be the optimal heat-treatment temperature for obtaining the most electroactive catalyst. Below 600 °C, more Fe–Nx active sites can be produced by increasing the pyrolysis temperature. However, above 600 °C, for example, at 700 °C, the structure of the catalyst will collapse. At the same time, undesirable secondary species will form, possibly leading to a reduced concentration of Fe–Nx moieties on the catalyst surfaces, as indicated by the XRD results below.
3.2. Electrode catalyst loading optimization with respect to the ORR activity
It is expected that increasing the non-noble metal catalyst loading inside the catalyst layer should be an effective way to improve ORR activity and performance.29 Therefore, we used the RDE technique to further investigate the loading effect on ORR activity in an O2-saturated 0.1 mol L−1 KOH solution. According to our current work, the Fe–N/C–TsOH catalyst obtained by pyrolysis at 600 °C would yield the highest ORR catalytic activity. So, to study the catalyst loading effect on ORR activity, Fe–N/C–TsOH-600 catalyst was used to prepare catalyst layers with different loadings (50.9, 101, 203.6, 305.4, and 407.2 mg cm−2). The resulting current–potential curves are shown in Fig. 4(a). Increasing the catalyst loading at the disk from 50.9 mg cm−2 to 407.2 mg cm−2 yields a much-improved half-potential (E1/2) and current density (id). In the catalyst loading range of 50 to 400 mg cm−2, the monotonic increase in ORR activity with increasing catalyst loading suggests that the electrochemically active Fe site density (or concentration) plays an effective role in the catalyst layer in terms of ORR activity enhancement.30 It is understandable that when the electrochemically active Fe site density rises, there are more neighboring catalyst active centers available for H2O2 to be further reduced to H2O, leading to a larger proportion of 4-electron pathway reactions in the whole ORR process through a (2 + 2)-electron series pathway.29 On the other hand, the ORR rate will be boosted as the loading increases, because the sites participate in the ORR process through an inner sphere mechanism to form O2-metal adducts such as O2–Fe–Nx. Another factor may be related to the catalyst layer thickness. The catalyst layer is thicker at a high catalyst loading than at a low one; thus, the H2O2 generated in the former case may have more time to stay within the catalyst layer to find another active site for further reduction before its release to the electrolyte, as shown in Fig. 4(b).3.3. Effect of KOH concentration
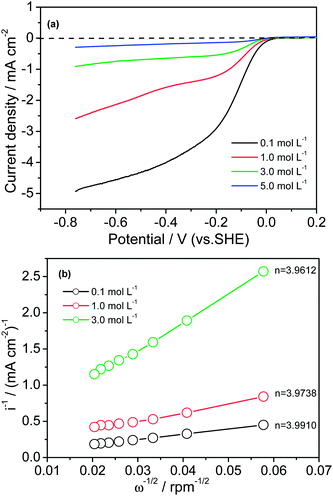
Electrocatalysis in alkaline media is a subject of growing industrial importance for fuel cells, since the ORR occurs more readily in alkaline electrolytes than in acidic or neutral electrolytes. For the purpose of practical applications, we studied our catalyst's activity toward the ORR in electrolytes containing various concentrations of KOH, to see how the catalyst activity changed with increasing KOH concentration. Fig. 5(a) presents the polarization curves for the ORR on an electrode in O2-saturated KOH electrolyte of various concentrations, from 0.1 to 5 mol L−1. We can see that:(1) The catalyst has a relatively well-defined diffusion-limiting current plateau in all tested KOH electrolytes in the potential range of −0.76 to −0.3 V, which means that the current density is limited by O2 diffusion from the solution to the electrode surface. Furthermore, the distribution of active sites on the electrode surface is uniform.
(2) Increased KOH concentration resulted in a large shift of the onset potential to a more negative value, with a large decrease in current densities in the kinetic, mixed, and diffusion-limiting regions. Clearly, the diffusivity and solubility of oxygen in KOH electrolytes can significantly contribute to the mass transfer effect of oxygen diffusion through the solution, thus affecting the current densities.
Fig. 5(b) shows the Koutecky–Levich plots obtained at −0.60 V for three different KOH concentrations (0.1, 1.0, and 3.0 mol L−1). From the slopes of the three lines, the overall electron numbers can be obtained, which are, respectively, 3.9910, 3.9738, and 3.9612. These values are fairly close to 4.0, indicating that the ORR catalyzed by Fe–N/C–TsOH catalyst is a 4-electron transfer process from O2 to H2O in all three KOH solution concentrations.
3.4. Electrocatalytic stability
The electrocatalytic stability of catalysts is an important parameter for their practical application. Fig. 6(a) shows the linear potential scan curves of Fe–N/C–TsOH-600 in O2-saturated 0.1 mol L−1 KOH at an electrode rotation speed of 1500 rpm after CV with different numbers of scans, as indicated. A potential cycling test was carried out between −0.8 and 0.3 V in N2-saturated 0.1 mol L−1 KOH with a potential scan rate of 50 mV s−1. The polarization curves were measured before and after 1500, 4800, and 6000 cycles in O2-saturated 0.1 mol L−1 KOH using a potential scan rate of 5 mV s−1 and an electrode rotation rate of 1500 rpm. A negative shift in both half-wave potential and current density during the first 1500 cycles of potential cycling is shown in Fig. 6(a). But the catalyst performance stabilizes during the last 4800 cycles, without further ORR activity change, indicating that the surface properties of the catalyst are maintained during cycling. Generally, there are two distinct performance degradation stages for a non-precious metal catalyst: an initial higher decay rate and a subsequent lower one. Therefore, the potential cycling method may be a promising protocol to quickly evaluate the stability of a non-precious metal catalyst. To clearly assess the catalyst's stability properties, we calculated the percentage of current density loss, iloss%, at different potentials, as summarized in Fig. 6(b). The iloss% is expressed as follows:| iloss% = (iini − i)/iini × 100% | (2) |
The iloss% of the ORR on the catalyst rises exponentially as the amount of potential cycling increases. This may indicate some intrinsic relationship between the active sites in the catalyst and the kinetics of the ORR. However, further experimental and modeling research is needed to clarify this relationship.
Furthermore, the Fe–N/C–TsOH-600 catalyst was leached in 0.5 mol L−1 H2SO4 to remove excess metals on the catalyst surface, then put it through a second heat treatment at 600 °C under N2 for 2 hours. Fig. 7(b) shows the polarization curves for oxygen reduction on Fe–N/C–TsOH catalysts subjected to different conditions. The measurements were conducted in O2-saturated 0.1 mol L−1 KOH using a potential scan rate of 5 mV s−1 and an electrode rotation rate of 1500 rpm. It is evident that all samples are catalytically active toward oxygen reduction. The re-pyrolyzed catalyst shows better catalytic activity than the others. Additionally, the onset potential of the ORR on Fe–N/C–TsOH-600 is as high as 50 mV. Leaching in 0.5 mol L−1 H2SO4 for a short while causes a positive shift in the onset potential of Fe–N/C–TsOH-600L and Fe–N/C–TsOH-600LH to 54 mV and 121 mV, respectively. It maybe that species less active toward the ORR, such as Fe3O4, dissolved in the 0.5 mol L−1 H2SO4 and at the same time increased the surface area of the catalyst. As well, re-pyrolysis is apt to form sites that are much more active and to allow uniform dispersion and stable distribution of the metal on Vulcan XC 72R, thereby improving the electrocatalytic activity of Fe–N/C–TsOH-600LH.
The complete set of oxygen reduction currents on Fe–N/C–TsOH in 0.1 mol L−1 KOH, detected at the disk electrode, is summarized in Fig. 7(a). Hydrogen peroxide produced in the course of the ORR was detected by utilizing the ring electrode in the collection mode. The potential of the ring electrode was set at 1.2 V, i.e., the potential at which H2O2 oxidation proceeds as a diffusion-limited process. Peroxide production (in %) is calculated from the ring-disk measurements, according to the following equation:31
| χH2O2 (%) = 100 [(2iring/N)/(idisk + iring/N)] | (3) |
3.5. Electrocatalytic selectivity in the presence of methanol
In a direct methanol fuel cell system, particularly with Pt-based fuel cell catalysts, methanol crossover to the cathode is one of the most significant problems because of the resulting mixed potential, which directly causes losses in efficiency and power density. Thus, it is desirable to develop electrocatalysts with a high selectivity towards oxygen reduction in a system containing methanol. We therefore also investigated ORR activity in the presence of methanol on Fe–N/C–TsOH-600 and Pt/C, and the effect of different methanol concentrations on the ORR performance of both catalysts. Fig. 8 presents the ORR curves of these two catalysts in O2-saturated 0.1 mol L−1 KOH containing 0, 0.5, 1.0, and 5.0 mol L−1 methanol, at an electrode rotation speed of 1500 rpm and room temperature. Clearly, the Pt/C catalyst favors the methanol oxidation reaction over the ORR, compared with Fe–N/C–TsOH-600. In methanol-free electrolyte, no methanol-oxidation current is found for either of the catalysts. However, when the methanol concentration is changed from 0 to 5 mol L−1, the onset potential shifts from 250 to −220 mV (Fig. 8(a)). The competitive adsorption of oxygen and methanol molecules on the surface of Pt/C results in a mixed potential. The electrode reaction was dominated by oxidation at high molecular concentrations, which limited the dissolved oxygen because Pt/C is active for both oxygen and methanol. In the case of Fe–N/C–TsOH-600, the ORR onset potential and current density change very little with or without the presence of methanol, up to 5.0 mol L−1 (Fig. 8(b)). At −2 mA cm−2, however, the potential shifts from −131 to −220 mV, indicating that species in a methanol solution within an alkaline medium disrupt the ORR. Nevertheless, our results show that in 0.1 mol L−1 KOH, this new material has a relatively higher selectivity towards the ORR in the presence of methanol than does Pt/C, although Fe–N/C–TsOH-600 does not have complete methanol tolerance.3.5. Morphology and structure of the prepared catalysts
Fig. 9 shows X-ray diffraction spectra of non-pyrolyzed and pyrolyzed Fe–N/C–TsOH catalysts (at 600 and 700 °C, respectively). The spectra exhibit the diffraction characteristics of the carbon support, with a broad shoulder in the range of 20° to 30° for 2θ. From Fig. 9 it can be seen that Fe–N/C–TsOH-RT shows quite strong diffraction peaks due to the crystalline nature of FeSO4·7H2O. These peaks disappear after high-temperature treatment. In their place, additional diffraction signals form at 600 and 700 °C, which may be due to the generation of metallic iron (α-Fe), iron carbide (Fe3C), and iron oxide-like magnetite (Fe3O4), as well as FeS, respectively.32 These results indicate that the structure of the Fe(II)–PPy precursor complex may have decomposed during the pyrolysis process, and those species could be types of ORR active sites, although with much less activity than is expected for Fe–Nx sites.33 It is notable that Fe–N/C–TsOH pyrolyzed at 700 °C exhibits very similar peaks to the catalyst pyrolyzed at 600 °C, except that: (1) the shape of these peaks of α-Fe, Fe3C, and Fe3O4 for Fe–N/C–TsOH-700 become much stronger and sharper than for Fe–N/C-600. Evidently, due to the formation of these less active species, the quantity of ORR active sites is reduced, thus resulting in less ORR activity for the Fe–N/C–TsOH-700;30 (2) when the samples further heat-treated at 700 °C, the diffraction signals of FeS were weakened, indicated that these FeS species maybe contribute to promote the ORR, somehow. So, to avoid the formation of additional species with less ORR activity, the thermal temperature should be below 700 °C for a Fe–N/C–TsOH precursor.To obtain information about the surface atomic composition of the pyrolyzed samples, XPS analysis was performed for unpyrolyzed samples and samples pyrolyzed at 600 and 700 °C. The broad scan of all three catalysts showed the peaks associated with C, N, O, S, and Fe. This result agrees with the EDX analysis.
The N 1s and S 2p core levels of the catalysts were recorded for both unpyrolyzed and pyrolyzed Fe–N/C–TsOH samples. Fig. 10(a)–(c) show the N 1s binding energy region, where the peaks of N 1s at 398.9, 400.5, and 401.3 eV can be attributed to pyridinic N, pyrrolic N, and graphitic N, respectively.34–36 From Fig. 10(a), the deconvolution of N signals for unpyrolyzed Fe–N/C–TsOH can be divided into two peaks, corresponding to pyridinic and pyrrolic N. When the thermal treatment temperature is increased to 600 °C, more of the pyridinic N transforms into pyrrolic N, such that at 600 °C, the fraction of pyrrolic N groups is the larger of the two; this catalyst exhibits higher activity than the unpyrolyzed catalyst, as was shown by the CV and RDE results in Fig. 1. This indicates that the pyrrolic N group is more active for oxygen reduction, and the catalyst pyrolyzed at 600 °C has more active sites (pyrrolic N) to facilitate oxygen adsorption. In addition, the preferred protonation of pyridinic sites may explain why pyrolysis at high temperatures leads to more pyrrolic N and less pyridinic N, resulting in more stable catalysts.37 Furthermore, when the Fe–N/C–TsOH samples were heat treated at 700 °C, deconvolution of the N signals gave three bands that could be assigned to pyridinic, pyrrolic, and graphitic N, respectively. The pyrrolic N peak is weaker than the one presented in Fig. 10(b), which agrees very well with earlier reports identifying pyrrolic N as responsible for the unique activity of Fe-containing catalysts.38
 | ||
| Fig. 10 XPS spectra of deconvoluted N 1s (a, c, and e) and S 2p (b, d, and f) for Fe–N/C-TsOH catalyst. (a) and (b) unpyrolyzed; (c) and (d) pyrolyzed at 600 °C; (e) and (f) pyrolyzed at 700 °C. | ||
Fig. 10(d) presents the S 2p spectra measured for the unpyrolyzed catalyst sample (Fe–N/C–TsOH). It can be seen that the catalyst without thermal treatment shows a large band at 167.0–171.0 eV, which can be assigned to the sulfate in the catalyst precursor.22,39 After pyrolysis at 600 °C (Fe–N/C–TsOH-600), the deconvolution of the S signals gave two bands with binding energies of 163.8 eV and 168.8 eV, respectively, as shown in Fig. 10(e). These peaks can be attributed to the binding sulfurs in C–S(n)–C (n = 1 or 2) bonds (60%) and oxidized –SO(n)– bonds (40%), which are expected to occur at the edges of carbon.40 Guo et al. found that the –C–S–C– structure is an important factor for optimizing ORR performance.20 This fact may explain why our catalyst pyrolyzed at 600 °C shows better catalytic ORR activity. It is believed that pyrolysis of the catalyst in the presence of sulfur could lead to mainly amorphous carbon, resulting in increased catalyst porosity and, in turn, enhanced catalyst performance.22,24 As well, the sulfur group doped into M–Nx–C might be helpful for entrapping M ions in an environment rich in pyrrole-type nitrogen. Therefore, it can be concluded that both sulfur doping and nitrogen doping of carbon play key roles in improving electrocatalytic activity for the ORR. For the samples pyrolyzed at higher temperatures, the S 2p peak can be fitted with four different sulfur moieties.41 The major contributions at binding energies around 163.8 eV (35%, lower 60%), 165.0 eV (30%), and 168.8 eV (30%) can be attributed to the binding sulfurs in C–S(n)–C (n = 1 or 2) bonds, conjugated –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S– bonds, and –SOn–. The remaining contribution at around 162.1 eV (5%) can be assigned to the reduced (–SH) sulfur moieties. The appearance of –C
S– bonds, and –SOn–. The remaining contribution at around 162.1 eV (5%) can be assigned to the reduced (–SH) sulfur moieties. The appearance of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S– and –SH reduced the amount of C–S–C, which is why Fe–N/C–TsOH-700 shows lower activity than the catalyst pyrolyzed at 600 °C. As mentioned above, both N and S play an important role in improving ORR activity, with pyrrolic N and C–S–C possibly serving as the ORR catalytic sites.
S– and –SH reduced the amount of C–S–C, which is why Fe–N/C–TsOH-700 shows lower activity than the catalyst pyrolyzed at 600 °C. As mentioned above, both N and S play an important role in improving ORR activity, with pyrrolic N and C–S–C possibly serving as the ORR catalytic sites.
4. Conclusions
Nitrogen/sulfur co-doped carbon materials were explored as non-precious metal catalysts for the ORR in alkaline media. They showed promising catalytic activity towards the ORR along a 4-electron transfer pathway in the overall reaction, and higher tolerance to methanol in comparison to commercial Pt/C catalyst in 0.1 mol L−1 KOH.The N and S dual-doped catalyst exhibited higher catalytic activity for oxygen reduction in alkaline media than one doped only with N, in terms of onset potential, half-wave potential, and diffusion-limited current density values. In addition, the catalytic activities were strongly dependent on the pyrolysis temperature used in catalyst synthesis, with the best ORR performance obtained at 600 °C. Instrumental analysis and XRD and XPS results all showed that 600 °C may be the optimal temperature for gaining more Fe–Nx active sites, where the pyrrolic N groups are the most active. Sulfur species structurally bound to carbon in the forms of C–S–C and oxidized –SOn– bonds play the key roles in improving ORR activity.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 91223202), the International Science & Technology Cooperation Program of China (no. 2011DFA73410), the Tsinghua University Initiative Scientific Research Program (no. 20101081907), and the National Key Basic Research Program of the China-973 Program (no. 2011CB013102).Notes and references
- R. Dillon, S. Srinivasan, A. S. Arico and V. Antonucci, J. Power Sources, 2004, 127, 112 CrossRef CAS PubMed.
- J.-L. Qiao, L. Xu, L. Ding, P.-H. Shi, L. Zhang, R. Baker and J.-J. Zhang, Int. J. Electrochem. Sci., 2013, 8, 1189 CAS.
- S. Liu, H. Zhang, Z. Xu, H. Zhong and H. Jin, Int. J. Hydrogen Energy, 2012, 37, 19065 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760 CrossRef CAS PubMed.
- S. Wang, D. Yu and L. Dai, J. Am. Chem. Soc., 2011, 133, 5182 CrossRef CAS PubMed.
- F. Charreteur, F. Jaouen and J. P. Dodelet, Electrochim. Acta, 2009, 54, 6622 CrossRef CAS PubMed.
- C.-W.-B. Bezerra, L. Zhang, H. Liu, K. Lee, A.-L.-B. Marques, E.-P. Marques, H. Wang and J. Zhang, J. Power Sources, 2007, 173, 891 CrossRef CAS PubMed.
- C.-H. Wang, H.-C. Hsu, S.-T. Chang, H.-Y. Du, C.-P. Chen, J.-C.-S. Wu, H.-C. Shih, L.-C. Chen and K.-H. Chen, J. Mater. Chem., 2010, 20, 7551 RSC.
- J.-S. Yu, M.-S. Kim and J.-H. Kim, Phys. Chem. Chem. Phys., 2010, 12, 15274 RSC.
- D. Xia, S. Liu, Z. Wang, G. Chen, L. Zhang, L. Zhang, S. Hui and J. Zhang, J. Power Sources, 2008, 177, 296 CrossRef CAS PubMed.
- C.-H. Wang, S.-T. Chang, H.-C. Hsu, H.-Y. Du, J.-C.-S. Wu, L.-C. Chen and K.-H. Chen, Diamond Relat. Mater., 2011, 20, 322 CrossRef CAS PubMed.
- B. Merzouguia, A. Hachimia, A. Akinpelu, S. Bukola and M. Shao, Electrochim. Acta, 2013, 107, 126 CrossRef PubMed.
- F. Jaouen, V. Goellne, M. Lefèvre, J. Herranz, E. Proietti and J.-P. Dodelet, Electrochim. Acta, 2013, 87, 619 CrossRef CAS PubMed.
- R. Jiang and D. Chu, J. Electrochem. Soc., 2000, 147, 4605 CrossRef CAS PubMed.
- D. Chu and R. Jiang, Solid State Ionics, 2002, 148, 591 CrossRef CAS.
- R. Holze, I. Vogel and W. Vielstich, J. Electroanal. Chem., 1986, 210, 277 CrossRef CAS.
- L. Yang, S. Jiang, Y. Zhao, L. Zhu, S. Chen, X. Wang, Q. Wu, J. Ma, Y. Ma and Z. Hu, Angew. Chem., Int. Ed., 2011, 50, 7132 CrossRef CAS PubMed.
- Z. Liu, F. Peng, H. Wang, H. Yu, J. Tan and L. Zhu, Catal. Commun., 2011, 16, 35 CrossRef CAS PubMed.
- D. Deak, E.-J. Biddinger, K.-A. Luthman and U.-S. Ozkan, Carbon, 2010, 48, 3635 CrossRef PubMed.
- H. Wang, X. Bo, Y. Zhang and L. Guo, Electrochim. Acta, 2013, 108, 404 CrossRef CAS PubMed.
- R. Ahmadi, M. K. Amini and J.-C. Bennett, J. Catal., 2012, 292, 81 CrossRef CAS PubMed.
- J. Qiao, L. Xu, L. Ding, L. Zhang, R. Baker, X. Dai and J. Zhang, Appl. Catal., B, 2012, 125, 197 CrossRef CAS PubMed.
- U. I. Kramm, I. Herrmann, S. Fiechter, G. Zehl, I. Zizak, I. Abs-Wurmbach, J. Radnik, I. Dorbandt and P. Bogdanoff, ECS Trans., 2009, 25, 659 CAS.
- I. Herrmann, U. I. Kramm, J. Radnik, S. Fiechter and P. Bogdanoff, J. Electrochem. Soc., 2009, 156, 1283 CrossRef PubMed.
- P.-H. Matter, L. Zhang and U.-S. Ozkan, J. Catal., 2006, 239, 83 CrossRef CAS PubMed.
- H.-J. Zhang, Q. Z. Jiang, L. Sun, X. Yuan and Z.-F. Ma, Electrochim. Acta, 2010, 55, 1107 CrossRef CAS PubMed.
- G. Hinds, NPL Report DEPC-MPE, 2005, vol. 19, p. 10 Search PubMed.
- C.-W.-B. Bezerra, L. Zhang, K. Lee, H. Liu, J. Zhang, Z. Shi, A.-L.-B. Marques, E.-P. Marques, S. Wu, S. Wu and J. Zhang, Electrochim. Acta, 2008, 53, 7703 CrossRef CAS PubMed.
- F. Charreteur, S. Ruggeri, F. Jaouen and J.-P. Dodelet, Electrochim. Acta, 2008, 53, 6881 CrossRef CAS PubMed.
- L. Wang, L. Zhang and J. Zhang, Electrochem. Commun., 2011, 13, 447 CrossRef CAS PubMed.
- B.-B. Blizanac, P.-N. Ross and N.-M. Markovic, Electrochim. Acta, 2007, 52, 2264 CrossRef CAS PubMed.
- A. Velazquez-Palenzuela, L. Zhang, L. Wang, P.-L. Cabot, E. Brillas, K. Tsay and J. Zhang, Electrochim. Acta, 2011, 56, 4744 CrossRef CAS PubMed.
- F. Jaouen and J.-P. Dodelet, Electrochim. Acta, 2007, 52, 5975 CrossRef CAS PubMed.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781 CrossRef CAS.
- G. Liu, X. Li, P. Ganesan and B.-N. Popov, Electrochim. Acta, 2010, 55, 2853 CrossRef CAS PubMed.
- G. Liu, X. Li, P. Ganesan and B.-N. Popov, Appl. Catal., B, 2009, 93, 156 CrossRef CAS PubMed.
- G. Wu, K. Artyushkova, M. Ferrandon, J. Kropf, D. Myers and P. Zelenay, ECS Trans., 2009, 25, 1299 CAS.
- G. Wu, Z. Chen, K. Artyushkova, F.-H. Garzon and P. Zelenay, ECS Trans., 2008, 16, 159 CAS.
- L. Zhu, D. Susac, M. Teo, K.-C. Wong, P.-C. Wong, R.-R. Parsons, D. Bizzotto, K. A.-R. Mitchell and S.-A. Campbell, J. Catal., 2008, 258, 235 CrossRef CAS PubMed.
- Y. Su, Y. Zhang, X. Zhuang, S. Li, D. Wu, F. Zhang and X. Feng, Carbon, 2013, 62, 296 CrossRef CAS PubMed.
- J.-P. Paraknowitsch, B. Wienert, Y. Zhang and A. Thomas, Chem. –Eur. J., 2012, 18, 15416 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |