Facile synthesis of WO3 with reduced particle size on zeolite and enhanced photocatalytic activity†
Abstract
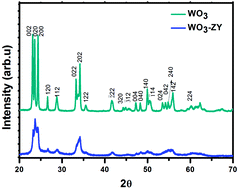
WO3 supported on zeolite-Y (WO3-ZY) was successfully synthesized by a facile impregnation method and well characterized by various techniques. The photocatalytic activity of the prepared catalysts was investigated for the degradation of Rhodamine B (RhB) under visible, UV and solar light irradiation. The enhanced photocatalytic activity was observed for the catalyst WO3-ZY, which may be due to the presence of more active sites that can adsorb a greater number of dye molecules. The TEM, FESEM and adsorption studies confirm that the WO3 supported on zeolite-Y has a very small particle size of about 8 nm compared with the bare WO3 at 97 nm. The efficient electron–hole pair separation and the role of active species were investigated by photoluminescence spectroscopy and the test of the effect of scavengers, respectively. The mechanism for the photocatalytic degradation of RhB was proposed and the pathway of RhB degradation was illustrated schematically.

 Please wait while we load your content...
Please wait while we load your content...