An improved stable Ti/Sb–SnO2 electrode with high performance in electrochemical oxidation processes†
Abstract
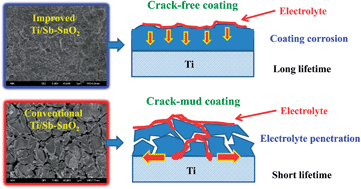
An improved Ti/Sb–SnO2 electrode was fabricated by inserting a specific Sb–SnO2 interlayer between the Ti substrate and the surface Sb–SnO2 coating. Characterization experiments including scanning electron microscopy (SEM), X-ray diffraction (XRD), energy dispersing spectrum (EDS), accelerated lifetime test, degradation experiment of an azo dye Acid Red G, and cyclic voltammetry (CV) measurement were performed to determine the effect of the interlayer. The results show that the coating on this new electrode is compact and crack-free. The distribution of Sn and Sb elements in the coating exhibits a gradient distribution from the bottom to the top. The high electrochemical oxidation capability of Ti/Sb–SnO2 did not decrease with the insertion of the interlayer. The improved electrode exhibited much higher electrode stability than the conventional Ti/SnO2 electrode. In a 0.5 M H2SO4 solution, at a current density of 200 mA cm−2, the accelerated lifetime of the conventional Ti/Sb–SnO2 was only 0.84 h, which is much lower that of the improved electrode (10.71 h). A possible reason for the electrode stability enhancement is attributed to the change in the electrode deactivation mechanism. In addition, practical service lifetimes were also estimated for the improved Ti/Sb–SnO2 in different media.

 Please wait while we load your content...
Please wait while we load your content...