Layer-controlled synthesis of graphene-like MoS2 from single source organometallic precursor for Li-ion batteries†
Jin Guoa,
Xiao Chena,
Yanjiao Yia,
Wenzhen Lib and
Changhai Liang*a
aLaboratory of Advanced Materials and Catalytic Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: changhai@dlut.edu.cn; Fax: +86-411-84986353; Tel: +86-411-84986353
bDepartment of Chemical Engineering, Michigan Technological University, Houghton, MI 49931, USA
First published on 26th March 2014
Abstract
Herein, we report an new approach to synthesis of graphene-like MoS2 flakes and tunable layers (from mono- to multi-layer) easily controlled by the thermal decomposition temperature of a single source (Mo(Et2NCS2)4). The approach opens a new way to controlled large-scalable synthesis of graphene-like transition metal sulfides for energy storage, nanoelectronics and optoelectronics.
Transition metal dichalcogenides have attracted great attention owing to their two-dimensional layer structure analogous to graphene. MoS2 is a typical inorganic layered material, in which each layer consists of a covalently bonded S–Mo–S hexagonal quasi-two-dimensional network,1,2 with weak van der Waals attraction between the layers. To date, a large number of researches have confirmed that the internal relationship between the shape and number of layers of MoS2 and its properties. Among them, few-layer MoS2 (especially mono-layer MoS2) has been successfully demonstrated as a promising material, which could complement graphene for a variety of applications such as lubricants, transistors,3,4 anode materials for lithium ion batteries,5,6 and photo/electro-catalysts7–10 due to its low cost, high chemical stability, good mechanical, distinctive electrical and optical properties.
However, the controlled synthesis of single and few layers MoS2 (named graphene-like MoS2) still remains highly challenging. Recently, significant efforts have been devoted to prepare mono- and few-layer MoS2. Novoselov et al.11 reported that few-layer MoS2 was synthesized by Scotch-tape assisted micromechanical exfoliation, but it was small scale and has poor repeatability. In addition, solution-phase production of MoS2 by exfoliation or hydrothermal method is often time-consuming.12–16 The synthetic routes also include epitaxial growth,17,18 intercalation assisted exfoliation,19–21 chemical vapor deposition (CVD),3,22 and sulfurization of molybdenum, phosphomolybdic acid, and molybdenum oxides.4,23 However, these current methods always involve high temperatures dangerous gases, including H2 and H2S, and complicated procedures. Therefore, developing simple and large scalable methods which graphene-like MoS2 can be produced in a controlled manner is highly needed.
The single source approach using stable organometallic precursors may be considered as an elegant and pertinent alternative to easily control the shape and layers. Indeed, the two-dimensional layer structure of MoS2 is anticipated to form through adjusting the metal–ligand interactions and decomposition temperatures. Thus, above-mentioned work shows the limits of the currently developed methods in terms of layer control, and further suggests that the rational design of precursors for layer number control is necessary to overcome these challenges. Here, we provide an effective approach to synthesis of the graphene-like MoS2 with controlled number of layers by thermal decomposition of a single source precursor tetrakis(diethylaminodithiocarbomato)molybdate(IV) (Mo(Et2NCS2)4). Compared with previous method, the controllable synthesis offers three advantages (i) the layer of the graphene-like MoS2 flakes can be easily controlled, (ii) the whole preparation process is very facial and has a good repeatability, and (iii) the few layer MoS2 flakes are highly dispersed which could offer high surface areas and has a better electrochemical performance than bulk MoS2. The resulting 2D graphene-like MoS2 flakes with uniform dispersion, which could offer high surface areas. As a consequence, the favorable graphene-like structure exhibit a storage capacity of 970 mA h g−1 in the initial cycles at a current density of 500 mA h g−1, and the cycling performance is much better than multi-layer MoS2 based anode.
The organometallic precursor Mo(Et2NCS2)4 was synthesized from Mo(CO)6 and bis(diethylthiocarbamoyl)disulfide following a modified procedure from the literature,24 as confirmed by the mass spectroscopy and X-ray single-crystal diffraction. As shown in Fig. S1 and Table S1,† the molecular weight of as-prepared Mo(Et2NCS2)4 is 689 g mol−1. The molecular structure of Mo(Et2NCS2)4 presumed from X-ray single-crystal diffraction is shown in the inserted of (Fig. 1) one Mo atom was coordinated with four diethylaminodithio-carbonato (S2N-(C2H5)2) ligands. The result is similar to the reference reported.24 Thermogravimetric analysis was applied to verify volatility characteristics (Fig. 1). The as-prepared precursor can be totally decomposed when the calcined temperature is above 374 °C. After the four decomposition steps, the residual black solid weight accounts for 25.5% of the initial weight. This is in good agreement with the calculated weight percent loss to produce pure MoS2 (23.5% calculated), the additional 2% material possibly attributed to decomposed ligand. The redundant sulfur, nitrogen and carbon in the precursor escaped as gaseous sulfur, hydrogen cyanide and ethylene.
 | ||
| Fig. 1 Molecular structure of Mo(Et2NCS2)4 precursor (a) and thermogravimetric analysis of the precursor (b). | ||
XRD patterns of the as-obtained product graphene-like MoS2 (GL-MoS2) and bulk MoS2 (B-MoS2) are shown in Fig. 2a. All the diffraction peaks in the pattern can be assigned as hexagonal MoS2 (JCPDS card no. 37-1492). The reflection peak at 2θ = 14.2° (002), corresponding to a d-spacing of 0.62 nm, indicates a well-staked layered structure. However, the MoS2 (002) reflection peak is not shown in the XRD patterns of the GL-MoS2 (320 °C, 400 °C, 600 °C), only two peaks at 2θ = 33.0°, 58.8° attributed to MoS2 (100) and (110) can be observed (as shown in Fig. 2a), which suggests the predominant formation of single layer or few layers MoS2.13 On the contrary, the sample of GL-MoS2 (800 °C) and B-MoS2 show a typical (002) reflection, which indicate a well-stacked layer structure.
The Raman spectra of samples excited by 532 nm line is shown in (Fig. 2b), which also confirmed the formation of MoS2 by thermal decomposition of a single source precursor Mo(Et2NCS2)4. Both B-MoS2 and GL-MoS2 exhibited sharp peaks at about 384 cm−1 (E12g) and 408 cm−1 (A1g) that were due to the first order Raman vibration modes within the S–Mo–S layer.25 Most strikingly, the E12g vibration and the A1g vibration of GL-MoS2 were stiffened (blue shift) with increasing annealing temperature. The result is consistent with a classical model for coupled harmonic oscillators that the E12g and A1g modes are expected to stiffen as additional layers, because the interlayer van der Waal interactions increase the effective restoring forces acting on the atoms.26 This result indicated the layers of GL-MoS2 increased with thermal-decomposition temperature increasing from 320 °C to 800 °C, which was consistent with the XRD result.
The morphology and microstructure of as-prepared GL-MoS2 and B-MoS2 were investigated via field-emission scanning electron microscope (FESEM) and transmission electron microscopy (TEM). As shown in Fig. 3a, the GL-MoS2 (400 °C) flakes are uniformly dispersed, which was main due to the spillover of redundant sulfur, nitrogen and carbon gas from the precursor Mo(Et2NCS2)4 during the calcination process, thus suppressing the sheet stacking together. However, the B-MoS2 possessed a large micrometer-sized scaled area, and the sheets were stacked together (as shown in Fig. 3b).
To further observe the microstructure, the as-prepared GL-MoS2 samples were characterized by TEM. Their TEM images demonstrated the coexistence of mono- and few-layer nanosheets, and the different layers of GL-MoS2 in Fig. 4a–d had been statistically analyzed. When the annealing temperature at 320 °C and 400 °C, the majority sheets were mono-layer, and the percentage of mono-layer can be reached about 76% and 70%, respectively. However, the percentage of mono-layer MoS2 was decreased but that of bilayer MoS2 was significant increased with increasing calcination temperature to 600 °C. Further increasing the temperature to 800 °C (as shown in Fig. 5d), the GL-MoS2 with some folded edges exhibited parallel lines corresponding to the different layers of MoS2 sheets (mainly layers = 6), which could be attributed to the crystallinity of MoS2 during higher temperature treatment. The structure of GL-MoS2 (800 °C) is similar to the reference B-MoS2 (Fig. 4e). This is consistent with the results of Raman and XRD analysis. Fig. 4f shows the varied layers with different temperature, which indicates that the two-dimensional layer structure of MoS2 can be well controlled by tuning the decomposition temperature ranges of the single source precursor Mo(Et2NCS2)4.
 | ||
| Fig. 4 TEM images of GL-MoS2 (320 °C) (a), GL-MoS2 (400 °C) (b), GL-MoS2 (600 °C) (c), GL-MoS2 (800 °C) (d), and B-MoS2 (e), and effect of decomposition temperatures on MoS2 stacking degree (f). | ||
Recently, we have also synthesized W(Et2NCS2)4 through a similar reaction of W(CO)6 and bis(diethylthiocarbamoyl)disulfide (Fig. S2†) and subsequently decomposed the single organometallic precursor to successfully prepare graphene-like WS2. Its 2-D structure has been evidenced by XRD and Raman characterizations, as shown in Fig. S3 and S4.† The results suggest that this method may open a new avenue to the synthesis of a wide range of graphene-like transition metal sulfides and make it convenient to the research of layers-dependent properties of transition metal sulfides.
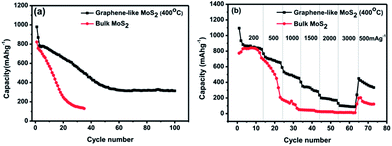
The charge/discharge characteristics were tested by galvanostatically cycling the cells based on the current density calculated by 500 mA g−1 in the potential range of 0–3 V vs. Li/Li+ (as shown in Fig. S5†). For GL-MoS2 (400 °C) at the first cycle, the charge (lithiation) and discharge (delithiation) capacities are 1093 and 905 mA h g−1, while that of B-MoS2 are 889 and 859 mA h g−1. As shown in (Fig. 5a), the capacity of the GL-MoS2 could be kept a stable storage capacity of about 330 mA h g−1 after 100 cycles at a current density of 500 mA g−1 between 0.01 and 3.0 V, which is much higher than the bulk-layer MoS2. The rate performance of the MoS2 is shown in Fig. 1. The GL-MoS2 exhibits a better rate performance than the solid ones. At the current densities of 200, 500, 1000, 1500, 2000, and 3000 mA g−1, the specific capacities of the graphene-like MoS2 are 890, 730, 540, 360, 235, and 130 mA h g−1, respectively. When the current density goes back to 500 mA g−1, the specific capacity of the graphene-like MoS2 returns to 450 mA h g−1, which is much better than bulk-MoS2. This mainly attributes to its optimal 2D graphene-like structure, which will enhance the contact area between MoS2 and electrolyte, shorten the lithium ion diffusion length, and facilitate the lithium ion ultrafast diffusion. Based on the excellent lithium-storage performance of single graphene-like MoS2 in our current study results, its long-time performance can be enhanced by deposition onto carbon materials to improve the structure stability and inhibit the MoS2 aggregation during the lithium ion insertion and extraction processes. As a result, it would be a promising anode material for energy storage applications in high-performance lithium ion battery.
In conclusion, we present an simple approach for synthesizing GL-MoS2 with controlled shape and number of layers by thermal decomposition of a single source precursor Mo(Et2NCS2)4 at different temperature. The obtained GL-MoS2 flakes with a relatively uniform dispersion, which exhibited high storage capacity of 970 mA h g−1 in the initial cycle at a current density of 500 mA g−1, and the cycling performance is much better than that of B-MoS2. This simple, large-scalable and reliable approach opens up a new avenue to preparation of graphene-like TMS in a controlled manner. The regular shape and controlled number of layers combined with the uniform dispersion make them a promising material for applications in energy storage devices as well as high-performance nanoelectronics and optoelectronics.
Experimental section
Synthesis of the GL-MoS2
GL-MoS2 was prepared by thermal decomposition of a single source precursor Mo(Et2NCS2)4. And single source precursor Mo(Et2NCS2)4 was prepared according to a reported method with some modifications.24 Typically, 1 g (3.8 mmol) Mo(CO)6 and 2.25 g (7.6 mmol) bis(diethylthiocarbamoyl)disulfide were dissolved in 30 mL acetone under an oxygen-free argon atmosphere. The solution was stirred and refluxed at 58 °C for 2.5 h, after cooling to room temperature naturally for 5 h. The violet precipitate were collected and washed with pentane. Finally, the product was dried under vacuum at 120 °C to evaporate off residual impurities.The single source precursor was heated to the various temperature (320, 400, 600, and 800 °C) at a rate of 10 °C min−1 under Ar and kept that temperature for 4 h. Then the graphene-like MoS2 can be obtained. The calcined temperature could be varied to obtain the desired results. To evaluate the electrochemical performances of the GL-MoS2, the B-MoS2 was prepared by thermal decomposition of the ammonium thiomolybdate ((NH4)2MoS4) under Ar.
Characterization
Thermogravimetric analysis (TGA) was analyzed by a SDTA851e apparatus at a heating rate of 10 °C min−1 in N2 atmosphere. XRD patterns were recorded with CuKα radiation on a D/MAX 2400 diffractometer, and the scan rate is 5° min−1 with a step of 0.02°. Raman measurements were obtained by an Invia/Reflex Lasser Micro-Raman spectroscope (Renishaw England) with a 532 nm Ar laser. FESEM images were obtained with an FEI Nova NanoSEM 450. TEM and HRTEM images were obtained with Philips CM200 electron microscope. The sample was dissolved in ethanol and the suspension was dropped onto a copper grid.Electrochemical measurements
The working electrodes were prepared by mixing 70 wt% active materials (MoS2), 20 wt% acetylene black (super-p) and 10 wt% poly(vinylidene fluoride) (PVDF) dissolved in N-methyl-2-pyrrolidinone. After coating the above slurry onto a copper foil current collector, it was dried under vacuum at 120 °C for 12 h and cut into pieces with a diameter of 14 mm before use. A Celgard 2300 membrane was used as a separator between the working electrode and the counter electrode (Li foil). The electrolyte was 1 M LiPF6 in the mixture of ethylene carbonate–dimethyl carbonate–diethyl carbonate (EC–DMC–DEC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v/v). The working electrode, the separator, the electrolyte, and the counter electrode, were assembled to a coin-type cell (2016) in an argon-filled glove box Unilab(1200/780). Galvanostatic charge/discharge cycles of the cells were conducted between 0.01 and 3.00 V on a LAND CT-2001A battery cycler (Wuhan, China) at room temperature.
1 v/v/v). The working electrode, the separator, the electrolyte, and the counter electrode, were assembled to a coin-type cell (2016) in an argon-filled glove box Unilab(1200/780). Galvanostatic charge/discharge cycles of the cells were conducted between 0.01 and 3.00 V on a LAND CT-2001A battery cycler (Wuhan, China) at room temperature.
Acknowledgements
We gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (21373038) and the Fundamental Research Funds for the Central Universities (DUT12YQ03).Notes and references
- S. Helveg, J. V. Lauritsen, E. Lægsgaard, I. Stensgaard, J. K. Nørskov, B. Clausen, H. Topsøe and F. Besenbacher, Phys. Rev. Lett., 2000, 84, 951 CrossRef CAS.
- J. V. Lauritsen, J. Kibsgaard, S. Helveg, H. Topsoe, B. S. Clausen, E. Laegsgaard and F. Besenbacher, Nat. Nanotechnol., 2007, 2, 53–58 CrossRef CAS PubMed.
- X. Wang, H. Feng, Y. Wu and L. Jiao, J. Am. Chem. Soc., 2013, 135, 5304–5307 CrossRef CAS PubMed.
- K. K. Liu, W. Zhang, Y. H. Lee, Y. C. Lin, M.-T. Chang, C. Y. Su, C. S. Chang, H. Li, Y. Shi and H. Zhang, Nano Lett., 2012, 12, 1538–1544 CrossRef CAS PubMed.
- Z. Wang, T. Chen, W. Chen, K. Chang, L. Ma, G. Huang, D. Chen and J. Y. Lee, J. Mater. Chem. A, 2013, 1, 2202–2210 CAS.
- K. Chang, W. Chen, L. Ma, H. Li, H. Li, F. Huang, Z. Xu, Q. Zhang and J.-Y. Lee, J. Mater. Chem., 2011, 21, 6251–6257 RSC.
- Y. Li, Y.-L. Li, C. M. Araujo, W. Luo and R. Ahuja, Catal. Sci. Technol., 2013, 3, 2214–2220 CAS.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575–6578 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Peng, Z. Meng, C. Zhong, J. Lu, W. Yu, Y. Jia and Y. Qian, Chem. Lett., 2001, 30, 772–773 CrossRef.
- H. S. Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059–4062 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan, G. Moriarty, J. Chen, J. Wang, A. I. Minett, V. Nicolosi and J. N. Coleman, Adv. Mater., 2011, 23(34), 3944–3948 CrossRef CAS PubMed.
- C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass and A. N. Marchenkov, Science, 2006, 312, 1191–1196 CrossRef CAS PubMed.
- P. W. Sutter, J.-I. Flege and E. A. Sutter, Nat. Mater., 2008, 7, 406–411 CrossRef CAS PubMed.
- A. Schumacher, L. Scandella, N. Kruse and R. Prins, Surf. Sci., 1993, 289, 595–598 CrossRef.
- R. Gordon, D. Yang, E. Crozier, D. Jiang and R. Frindt, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65 Search PubMed.
- M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. Li and S. Jin, J. Am. Chem. Soc., 2013, 135, 10274–10277 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- H. Liu, D. Su, R. Zhou, B. Sun, G. Wang and S. Z. Qiao, Adv. Energy Mater., 2012, 2(8), 970–975 CrossRef CAS PubMed.
- M. Decoster, F. Conan, J. Guerchais, Y. Le Mest, J. Sala Pala, J. Jeffery, E. Faulques, A. Leblanc and P. Molinié, Polyhedron, 1995, 14, 1741–1750 CrossRef CAS.
- C. Chang and S. Chan, J. Catal., 1981, 72, 139–148 CrossRef CAS.
- T. Li and G. Galli, J. Phys. Chem. C, 2007, 111, 16192–16196 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Mass spectrum, molecular structure and the brief crystallographic data and data collection of Mo(Et2NCS2)4. Molecular structure of W(Et2NCS2)4, XRD pattern and Raman spectrum of graphene-like WS2. CCDC 992256 contains the supplementary crystallographic data for this paper. See DOI: 10.1039/c4ra01318b |
| This journal is © The Royal Society of Chemistry 2014 |