Significant enhancement of visible up-conversion emissions of Y2O2S:Er3+ phosphors by Mn2+ sensitizing under 1550 nm excitation
Abstract
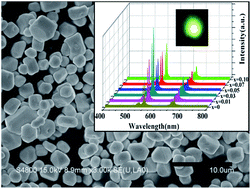
A novel up-conversion (UC) emission route of Er3+ by Mn2+ sensitizing in a Y2O2S:Er3+ phosphor prepared via a high-temperature solid-state reaction method is reported here. This methodology resulted in a significant enhancement of visible up-conversion emission by 1550 nm laser diode excitation. By co-doping Mn2+, the visible (red and green) UC emission intensity, which is assigned to the transitions of 4F9/2 → 4I15/2 (670 nm), 4S3/2 → 4I15/2 (547 nm), 2H11/2 → 4I15/2 (528 nm) of Er3+, was 2 to 3 times higher than that of the Mn2+-free one. As the concentration of Mn2+ was increased, the visible UC emission intensity was enhanced and then reduced. The phosphor powders were well crystallized in a hexagonal shape with an average size of ca. 2 μm. Based on the results of the luminescence spectra, energy matching conditions and three-photon process dependence on excitation power, a possible UC mechanism is proposed. The proposed sensitizing route may lead to a promising step towards high-intensity UC emissions of rare-earth ion doped phosphors.

 Please wait while we load your content...
Please wait while we load your content...