Rapid-flux-solvent-atmosphere method for tailoring the morphology of titania substrates over a large area via direct self-assembly of block copolymers†
Abstract
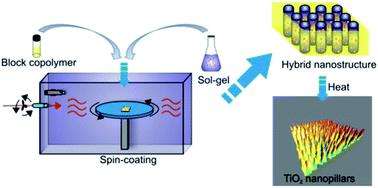
A fast method to direct the self-assembly of block copolymers loaded with metal oxide precursors in specific domains is demonstrated. It consists of performing spin-coating in a controlled atmosphere under a flux of solvent vapors (rapid-flux-vapor-atmosphere). Nanostructured thin films of hybrid composites and regular titania substrates, well oriented over a large area, are rapidly obtained.

 Please wait while we load your content...
Please wait while we load your content...