Facile fabrication of Chinese lantern-like MnO@N–C: a high-performance anode material for lithium-ion batteries†
Abstract
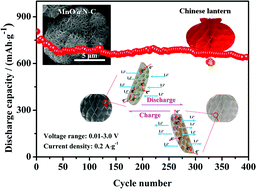
Chinese lantern-like MnO coated with N-doped C (MnO@N–C) was fabricated from Chinese lantern-like MnCO3 coated with polydopamine (MnCO3@PDA) precursor that was calcined at 600 °C for 5 h under an N2 atmosphere. MnO@N–C was then investigated as an anode for lithium-ion batteries. Structural characterization results indicate that MnO@N–C comprised numerous nanoplates with a thickness of ∼35 nm. The nanoplates consisted of MnO nanoparticles (∼15 nm) that were homogeneously embedded within the N-doped C (N–C) matrix. The uniformly embedded MnO nanoparticles can realize a high electrochemical utilization of the material to generate a high specific capacity. The N–C matrix and voids between the nanoplates can minimize strain, thereby maintaining the structural stability of the MnO@N–C electrode during the discharge/charge process, enabling improved cyclic performance. Additionally, the MnO@N–C nanoplates with high specific surface area in the Chinese lantern-like framework can shorten the path diffusing length of the lithium ions and the N–C matrix can provide efficient electrical integrity to the electrode, which can enhance the rate capability. Consequently, the obtained MnO@N–C exhibits a high reversible specific capacity of 810 mA h g−1 at 0.2 A g−1, favorable cyclic stability of 640 mA h g−1 after 400 cycles, and excellent rate capability of 451 mA h g−1 at 1 A g−1 and 285 mA h g−1 at 4 A g−1.

 Please wait while we load your content...
Please wait while we load your content...