Bio-based tetrafunctional crosslink agent from gallic acid and its enhanced soybean oil-based UV-cured coatings with high performance
Abstract
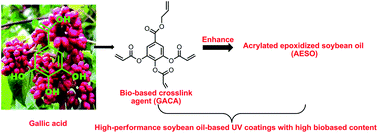
The utilization of soybean oil-based UV coatings depends on the introduction of petroleum-based comonomers or crosslink agents. Thus, in this paper, a bio-based crosslink agent (GACA) for UV curable coatings was synthesized from gallic acid and its chemical structure was confirmed by FT IR, 1H NMR and 13C NMR. Crosslinked networks with high biobased content of more than 88% were obtained after co-photopolymerization between acrylated epoxidized soybean oil (AESO) and GACA. The thermal, mechanical and coating properties of these GACA crosslinked AESO networks were investigated and a commonly used crosslink agent triallyl isocyanurate (TAIC) was used as the control. GACA exhibited more functional groups and better copolymerization with AESO than TAIC, resulting in the higher gel content, crosslink density, tensile strength and modulus as well as much better coating properties (reflected by the higher pencil hardness, better wear resistance and adhesion) of GACA crosslinked AESO networks than TAIC crosslinked AESO networks. These results indicated that GACA exhibited great potential to replace petroleum-based crosslink agents such as TAIC, and high-performance soybean oil-based UV-cured coatings with high biobased content could be achieved after introducing GACA.

 Please wait while we load your content...
Please wait while we load your content...