Ruthenium catalyzed regioselective coupling of terminal alkynes, amine and carbon dioxide leading to anti-Markovnikov adducts†
Rahul A. Watile and
Bhalchandra M. Bhanage*
Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai, 400 019, India. E-mail: bm.bhanage@gmail.com; bm.bhanage@ictmumbai.edu.in; Fax: +91 22 3361 1020; Tel: +91 22 3361 1111/2222
First published on 14th May 2014
Abstract
RuCl2(p-cymene)/DPPE catalyzed addition of secondary amines and CO2 to terminal alkynes affording anti-Markovnikov adducts of vinyl carbamates in good yield with excellent regioselectivity is reported. The catalyst, consisting of labile p-cymene displays very high regioselectivity towards the anti-Markovnikov adducts and was applicable to a variety of aliphatic/aromatic alkynes and secondary amines.
In recent years, the use of carbon dioxide (CO2) for the production of industrially important intermediates has attracted much more attention under the current background of the increase of emissions of CO2.1,2 However, the activation of CO2, a very stable molecule, is one of the biggest challenges in chemistry. Transition metal catalyzed transformation of CO2 into vinyl carbamates, or enol carbamates is one of the attractive routes in CO2 chemistry. Vinyl carbamates or enol carbamates are important building blocks, possessing wide application in agrochemicals, pharmaceuticals and are also essential monomers for transparent polymers and varnishes.3a,b
There are several synthetic pathways described for the preparation of vinyl carbamates in the literature (Scheme 1). Conventionally, alkyl carbamates synthesized via reaction of an amines with chloroformates,4a,b the dehydrohalogenation of α-halogeno,5a,b β-halogenoalkyl carbamates6 and addition of amines to vinyl chloroformates (VOC-Cl).7a,b A reaction of VOC-Cl with an amine is mostly used for the preparation of vinyl carbamates (Scheme 1).7b These traditional synthetic methods are associated with several limitations including use of poisonous carbonyl source, use of toxic and difficult to handle intermediates, multiple reaction steps, low atom efficiency with a stoichiometric use of reagents which result in the formation of chemical waste. There are several efforts have been made to replace these toxic reagents by catalytic incorporation of CO2 into organic substrate for their functionalization.8a–c
Few groups have reported ruthenium based catalytic protocols for the synthesis of vinyl carbamates using carbon dioxide as C1 source like RuCl2(norbornadiene)(pyridine)2, [RuCl2(norbornadiene)]n, Ru3(CO)12, RuCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3H2O,9a,b bis-(η5-cyclooctadienyl)ruthenium, Ru-(COD)(COT)-tertiary phosphine and [RuCl2(C5H5N)4], [RuCl2(6-C6H6)(PMe3)].10a,b Despite of their potential utility, the above methods suffer from one or more drawbacks like lower yield and poor regioselectivity, requirement of expensive, air sensitive and toxic ligands which limits their application. Also, generality of the protocol has not been explored with respect to the structural and electronic variation of alkynes as well as amines. Therefore, there is a need to develop an active, stable, regioselective catalyst for the one pot synthesis of vinyl carbamates from carbon dioxide, amines and alkynes which could be worked efficiently under mild reaction condition, is a subject of the present work.‡
3H2O,9a,b bis-(η5-cyclooctadienyl)ruthenium, Ru-(COD)(COT)-tertiary phosphine and [RuCl2(C5H5N)4], [RuCl2(6-C6H6)(PMe3)].10a,b Despite of their potential utility, the above methods suffer from one or more drawbacks like lower yield and poor regioselectivity, requirement of expensive, air sensitive and toxic ligands which limits their application. Also, generality of the protocol has not been explored with respect to the structural and electronic variation of alkynes as well as amines. Therefore, there is a need to develop an active, stable, regioselective catalyst for the one pot synthesis of vinyl carbamates from carbon dioxide, amines and alkynes which could be worked efficiently under mild reaction condition, is a subject of the present work.‡
Low-valent ruthenium complexes have proven to be excellent catalysts for this transformation. The features of [RuCl2(p-cymene)]2, complex to spontaneously form a Ru-vinylidene in the presence of a terminal alkyne were tempted us to develop a system able to catalyze vinyl carbamates synthesis using carbon dioxide, secondary amines and alkynes.11a–c
Continuing our efforts towards the development of a new facile protocol for incorporation of CO2 into organic chemicals,12 here, we employed the RuCl2(p-cymene)/DPPE as an efficient and highly active catalyst for regioselective synthesis of vinyl carbamates using carbon dioxide, secondary amines and alkynes (Scheme 2).
In order to optimize the reaction conditions, initially the reactions of secondary amine and CO2 to terminal alkynes were chosen as the model reaction. Various reaction parameters such as catalyst screening, catalyst loading, effects of solvents, and effect of CO2 pressure, reaction temperature and time were investigated and the results obtained are summarized in Table 1 and 2.
| Entry | Catalyst (loading mol%) | Yieldb (%) | Selectivity (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b) 2b) |
|---|---|---|---|
a Reaction conditions: phenyl acetylene (2 mmol), diethyl amine (4 mmol), metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio (1 ligand ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ACN (10 mL), CO2 pressure (5 MPa), temp. (80 °C), time (24 h).b Yield based on GC analysis. 1), ACN (10 mL), CO2 pressure (5 MPa), temp. (80 °C), time (24 h).b Yield based on GC analysis. |
|||
| Catalyst screening | |||
| 1 | RuCl3/(TPPTS)3 (1%) | 20 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 2 | RuCl2/(TPPTS)3/SILPC (1%) | 30 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| 3 | Ru(acac)3 (1%) | 17 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 4 | [Ru(TMHD)3] (1%) | 50 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 5 | RuCl3–EDTA (1%) | Trace | — |
| 6 | [RuCl2(p-cymene)]2 (1%) | 62 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 7 | [RuCl2(p-cymene)]2/PPh3 (1%) | 70 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
| 8 | [RuCl2(p-cymene)]2/DPPE (1%) | 78 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 9 | [RuCl2(p-cymene)]2/DPPB (1%) | 75 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
| Catalyst Loading | |||
| 10 | [RuCl2(p-cymene)]2/DPPE (0.3%) | 43 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
| 11 | [RuCl2(p-cymene)]2/DPPE (0.6%) | 64 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
| 12 | [RuCl2(p-cymene)]2/DPPE (1.5%) | 78 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| S. no. | Solvent | Temp. (°C) | CO2 (MPa) | Time (h) | Yieldb (%) | Selectivity (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b) 2b) |
|---|---|---|---|---|---|---|
| a Reaction conditions: phenyl acetylene (2 mmol), diethyl amine (4 mmol), catalyst (1 mol%), DPPE (1 mol%), ACN (10 mL), CO2 pressure (5 MPa), temp. (80 °C), time (24 h).b Yield based on GC analysis. | ||||||
| Effect of solvent | ||||||
| 1 | Toluene | 80 | 5 | 24 | 30 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 2 | DMF | 80 | 5 | 24 | 15 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 3 | THF | 80 | 5 | 24 | 49 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 4 | ACN | 80 | 5 | 24 | 78 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 5 | 1,4-Dioxane | 80 | 5 | 24 | 21 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 6 | Glycerol | 80 | 5 | 24 | 50 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 7 | Water | 80 | 5 | 24 | 10 | — |
| Effect of temperature | ||||||
| 8 | ACN | 60 | 5 | 24 | 38 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 9 | ACN | 100 | 5 | 24 | 54 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| Effect of CO2 pressure | ||||||
| 10 | ACN | 80 | 3 | 24 | 47 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 11 | ACN | 80 | 4 | 24 | 70 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 12 | ACN | 80 | 7 | 24 | 80 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
| Effect of time | ||||||
| 13 | ACN | 80 | 5 | 12 | 62 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 14 | ACN | 80 | 5 | 18 | 69 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 15 | ACN | 80 | 5 | 30 | 80 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
Firstly, we screened various metal complexes such as RuCl2/(TPPTS)3, RuCl2/(TPPTS)3/SILPC and β-diketonate complexes like Ru(acac)3, [Ru(TMHD)3]12a and RuCl3–EDTA (Table 1, entries 1–5). It was observed that the complexes like RuCl2/(TPPTS)3, RuCl2/(TPPTS)3/SILPC gave lower conversion of desired product (up to 30%). Whereas, β-diketonate complexes gave 17% yield. The complexes involving N-containing ligand, RuCl3–EDTA was found to be ineffective (Table 1, entry 5). Next we screened RuCl2(p-cymene) as a catalyst which furnished 62% conversion of desired Z-isomer of the product. This increase in yield by using RuCl2(p-cymene) precursor encouraged us to engage different phosphine ligands. Various phosphine containing ligands were screened for addition reactions of secondary amine and CO2 to terminal alkynes. The best result was obtained by using DPPE as a ligand [DPPE = 1,1-bis(diphenylphosphino)ethane] (78%), while other ligands were found to give moderate yield of desired product (Table 1, entries 7 and 9). The RuCl2(p-cymene)/DPPE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) was found to be the best catalyst providing good yield (78%) of vinyl carbamate with an excellent Z/E ratio of 93
1 ratio) was found to be the best catalyst providing good yield (78%) of vinyl carbamate with an excellent Z/E ratio of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 (Table 1, entry 8). The reactivity trend could results from the fact that there exist a labile p-cymene which could have displayed a very high regioselectivity towards the anti-Markovnikov adducts of alkenyl carbamates.
07 (Table 1, entry 8). The reactivity trend could results from the fact that there exist a labile p-cymene which could have displayed a very high regioselectivity towards the anti-Markovnikov adducts of alkenyl carbamates.
In transition metal-catalyzed reactions, the amount of catalyst employed proves to be an important aspect, considering this, the efforts were made to determine the optimum loading of the catalyst. We studied the catalyst loading ranging from 0.3 to 1.5 mol%, where increase in initial catalyst concentration up to 1.0 mol% has increased the yield of desired product (Table 1, entries 10–12) while further increase in the amount of catalyst had no profound effect on the yield of the desired product as well as selectivity (Table 1, entry 12).
The effect of different solvents on the reaction system was investigated and it was observed that nature of solvent affected the conversion of the reaction (Table 2, entries 1–7). The solvents like toluene, N,N-dimethylformamide (DMF), tetrahydrofuran (THF), 1,4-dioxane and acetonitrile (ACN) were screened for the standard reaction. However, among all the solvents screened ACN was found to furnish good yield and regioselectivity (Table 2, entry 4).
To examine the effect of reaction temperature on the yield of vinyl carbamates synthesis, the catalytic reaction of phenylacetylene, diethylamine and CO2 were studied at different reaction temperature ranging from 60–100 °C (Table 2, entries 8 and 9). It was observed that at 60 °C the yield of the desired product was low whereas with increase in reaction temperature up to 80 °C, the yield and selectivity of vinyl carbamate towards the Z-isomer (2a) was found to increase in 24 h. Further increase in the temperature to 100 °C resulted in decreased yield and selectivity was observed (Table 2, entry 9).
The influence of CO2 pressure for vinyl carbamates synthesis was then investigated. It was observed that increasing the pressure from 4 MPa to 5 MPa resulted increase in the yield and selectivity of the Z-isomer (Table 2, entries 10–12). Further increase in the pressure to 7 MPa had no profound effect on the reaction yield and selectivity (Table 2, entry 12). Hence, the best optimized reaction parameters for regioselective synthesis of vinyl carbamates were phenylacetylene, diethylamine and carbon dioxide are: Ru[Cl2(p-cymene)]2 (1.0 mol%), DPPE (1 mol%), solvent (ACN, 10 mL), diethylamine (4 mmol), CO2 pressure (5 MPa), temperature 80 °C, time 24 h.
With these optimized reaction conditions in hand, we investigated the scope and generality of the developed protocol for the synthesis of variety of vinyl carbamates. Various alkynes and secondary amines with different steric and electronic properties were screened (Table 3, entries 1–10). The addition reaction of diethyl amine and CO2 to phenylacetylene under the optimized reaction conditions providing a 78% isolated yield of the [(diethylcarbamoyl)oxy]styrene and (Z)-β-[(diethylcarbamoyl)oxy]styrene isomer with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 selectivity and along with small amount of dimmer of phenyl acetylene as biproduct. Dibutyl amine was also found to react efficiently with phenyl acetylene providing an excellent selectivity of (Z)-β-[(dibutylcarbamoyl)oxy]styrene isomer up to 91%. Alicyclic amines like morpholine and piperidine were found to provide good yield and selectivity of [(morpholinocarbamoyl)oxy]styrene and [(piperidinocarbamoyl)oxy] styrene isomer respectively (Table 3, entries 3–5). Further we screened N-methyl-1-phenylmethanamine and diallylamine and were found to furnish good yield and selectivity toward the formation of desire product (Table 3, entries 6 and 7).
07 selectivity and along with small amount of dimmer of phenyl acetylene as biproduct. Dibutyl amine was also found to react efficiently with phenyl acetylene providing an excellent selectivity of (Z)-β-[(dibutylcarbamoyl)oxy]styrene isomer up to 91%. Alicyclic amines like morpholine and piperidine were found to provide good yield and selectivity of [(morpholinocarbamoyl)oxy]styrene and [(piperidinocarbamoyl)oxy] styrene isomer respectively (Table 3, entries 3–5). Further we screened N-methyl-1-phenylmethanamine and diallylamine and were found to furnish good yield and selectivity toward the formation of desire product (Table 3, entries 6 and 7).
| Entry | Alkynes (R1) | Secondary amines (R2) | Yieldb (%) | Selectivity 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
|---|---|---|---|---|
| a Reaction conditions: alkynes (2 mmol), secondary amine (4 mmol), catalyst (1 mol%), DPPE (1 mol%), ACN (10 mL), CO2 pressure (5 MPa), temp. (80 °C), time (24 h).b Isolated yield. | ||||
| 1 | 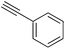 |
 |
78 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 2 |  |
 |
61 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
| 3 |  |
 |
47 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 4 |  |
 |
63 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 5 |  |
 |
49 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 6 |  |
 |
87 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 03 03 |
| 7 |  |
 |
84 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02 02 |
| 8 |  |
Et2NH | 63 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
| 9 |  |
Et2NH | 57 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 10 |  |
Et2NH | 48 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
The present catalytic system was also worked well with the electron donating and withdrawing aromatic substituted alkynes providing good conversions with excellent selectivity (Table 3, entries 8 and 9). Moreover, we also studied the aliphatic alkyne such as 1-hexyne; reacted under the present conditions to afford moderate yield of [(diethylcarbamoyl) oxy]hex-1-ene (Table 3, entry 10). Thus, improved yield and excellent selectivity was observed for various vinyl carbamates under the developed catalytic protocol as compared to earlier reports.
The plausible mechanism of addition reactions of secondary amine and CO2 to terminal alkynes for vinyl carbamate synthesis was shown in Scheme 3. Firstly, the rearrangement of ruthenium complex of type (I) into metal derivatives of type (II) takes place, which show electrophilic behavior of the carbon atom bonded to the metal center. Subsequent addition of carbamates to the electrophilic carbon of this vinylidene–ruthenium molecule (II) to give the intermediate (III).13 Subsequently, the ammonium cation [(R2)2NH2+] formed during the course of reaction which protonates the Ru metal followed by classical reductive elimination from (IV), leading to the enol carbamate. An alternative route could be protonolysis of the Ru–C bond (III) followed by 1–2 shift of hydrogen atom giving intermediate (VI), which on further de-coordination afford desired product.13b
In summary, RuCl2(p-cymene)/DPPE catalytic system has shown to be an efficient transition-metal catalyzed process for the synthesis of alkenyl carbamates via three-component addition of secondary amines and CO2 to terminal alkynes. The characteristic feature of present catalytic protocol is the high regioselectivity giving the anti-Markovnikov adducts in good to excellent yield. The different alkynes and secondary amines with different steric and electronic properties were explored for synthesis of alkenyl carbamates. The catalyst is highly stable and shows an excellent catalytic activity under optimized condition make it an ideal catalyst for regioselective synthesis of vinyl carbamate.
Acknowledgements
The author (R. A. Watile) is greatly thankful to Council of Scientific and Industrial Research (CSIR, India) for providing financial support (CSIR-SRF).References
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed; P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1995, 95, 259 CrossRef; M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81 Search PubMed.
- P. T. Anastas and R. L. Lankey, Green Chem., 2000, 2, 289 RSC; A. J. Hunt, E. H. K. Sin, R. Marriott and J. H. Clark, ChemSusChem., 2010, 3, 306 CrossRef CAS PubMed; T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef PubMed.
- (a) S. Boivin, A. Chettouf, P. Hemery and S. Boileau, Polym. Bull., 1983, 9, 114 CrossRef CAS; (b) R. J. Khur and H. W. Dorough, Carbamate insecticide: chemistry, biochemistry and toxicology, CRS Press, Cleveland OH, 1976 Search PubMed.
- (a) S. I. Murahashi, Y. Mitsue and K. Ike, J. Chem. Soc., Chem. Commun., 1987, 125 RSC; (b) S. Cenini, M. Pizzotti, C. Crotti, F. Porta and G. La Monica, J. Chem. Soc., Chem. Commun., 1984, 1286 RSC.
- (a) R. A. Olofson, G. P. Wooden and J. T. Marks, Eur. Pat. 104984, Chem. Abstr., 1984, 101, 190657; (b) B. R. Franco-Filipasic and R. Patarcity, Chem. Ind., 1969, 8, 166 Search PubMed.
- C. G. Overberger, H. Ringsdorf and N. Weinshenker, J. Org. Chem., 1962, 27, 4331 CrossRef CAS.
- (a) R. A. Olofson, B. A. Bauman and D. J. Vancowicz, J. Org. Chem., 1978, 43, 752 CrossRef CAS; (b) R. A. Olofson, R. C. Schnur, L. Bunes and J. P. Pepe, Tetrahedron Lett., 1977, 1567 CrossRef CAS.
- (a) S. Inoue and N. Yamazaki, Organic and bio-organic chemistry of carbon dioxide, Kodansha, Tokyo, 1982 Search PubMed; (b) A. Aresta and G. Forti, Carbon dioxide as a source of carbon; Reidal: NATO ASIseries C 206, 1987; (c) D. Walther, H. Schonberg, E. Dinjus and J. S. Sieler, J. Organomet. Chem., 1987, 334, 377 CrossRef CAS.
- (a) Y. Sasaki and P. H. Dixneuf, J. Chem. Soc., Chem. Commun., 1986, 790 RSC; (b) R. Mahe, Y. Sasaki, C. Bruneau and P. H. Dixneuf, J. Org. Chem., 1989, 54, 1518 CrossRef CAS; (c) C. Bruneau and P. H. Dixneuf, J. Mol. Catal., 1992, 74, 97 CrossRef CAS; (d) H. J. Reich, R. C. Holtan and S. L. Borkowsky, J. Org. Chem., 1987, 52, 314 CrossRef.
- (a) T.-A. Mitsudo, Y. Hori, Y. Yamakawa and Y. Watanabe, Tetrahedron Lett., 1987, 28, 4417 CrossRef CAS; (b) M. Rohr, C. Geyer, R. Wandeler, M. S. Schneider, E. F. Murphy and A. Baiker, Green Chem., 2001, 3, 123 RSC.
- (a) F. Simal, D. Jan, L. Delaude, A. Demonceau, M.-R. Spirlet and A. Noels, Can. J. Chem., 2001, 79, 529 CrossRef CAS; (b) D. Enders, K. Breuer, G. Raabe, J. Runsink, H. Teles, J.-P. Melder, K. Ebel and S. Brode, Angew. Chem., Int. Ed. Engl., 1995, 34, 1021 CrossRef CAS; (c) K. Melis, P. Samulkiewicz, J. Rynkowski and F. Verpoort, Tetrahedron Lett., 2002, 43, 2713 CrossRef CAS.
- (a) Y. P. Patil, P. J. Tambade, N. S. Nandurkar and B. M. Bhanage, Catal. Commun., 2008, 9, 2068 CrossRef CAS PubMed; (b) Y. P. Patil, P. J. Tambade, S. R. Jagtap and B. M. Bhanage, J. Mol. Catal. A: Chem., 2008, 289, 14 CrossRef CAS PubMed; (c) R. A. Watile, D. B. Bagal, K. M. Deshmukh, K. P. Dhake and B. M. Bhanage, J. Mol. Catal. A: Chem., 2011, 351, 196 CrossRef CAS PubMed; (d) R. A. Watile, D. B. Bagal, Y. P. Patil and B. M. Bhanage, Tetrahedron Lett., 2011, 52, 6383 CrossRef CAS PubMed; (e) D. B. Nale, S. Rana, K. M. Parida and B. M. Bhanage, Appl. Catal., A, 2014, 469, 340 CrossRef CAS PubMed; (f) R. A. Watile, K. M. Deshmukh, K. P. Dhake and B. M. Bhanage, Catal. Sci. Technol., 2012, 2, 1051 RSC.
- (a) M. I. Bruce and A. G. Swincer, Adv. Organomet. Chem., 1983, 22, 59 CrossRef CAS; (b) M. S. Brookhart, J. R. Tucker and G. R. Husk, J. Am. Chem. Soc., 1983, 105, 258 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, characterization data of catalyst. See DOI: 10.1039/c4ra03836c |
| ‡ Experimental All chemicals and reagents were purchased from firms of repute with their highest purity available and were used without further purification. [RuCl2(p-cymene)]2 precursor and phosphine ligands were purchased from Sigma-Aldrich. The reaction mixture was analyzed by GC (Perkin-Elmer, Clarus 400) equipped with a flame ionization detector (FID) and a capillary column (Elite-1, 30 m × 0.32 mm × 0.25 μm). The crude product was purified by column chromatography on silica gel (eluting with 80 General procedure for synthesis of vinyl carbamate from CO2 In a typical experimental procedure, the alkynes (2 mmol), secondary amine (4 mmol), [RuCl2(p-cymene)]2 (1 mol%), DPPE (1 mol%) and ACN (10 mL) were charged into a 100 mL stainless steel autoclave with a mechanical stirrer at room temperature. The autoclave was flushed with carbon dioxide and reaction mixture was then pressurized to 5 MPa of CO2 pressure; the reactor was heated to 80 °C and stirred for 24 h at 600 rpm. After completion of reaction, the reactor was cooled to room temperature and the remaining carbon dioxide was carefully vented and then the reactor was opened. The crude product which was then purified by column chromatography on silica gel (100–200 mesh size), with petroleum ether–ethyl acetate (PE–EtOAc, 80 |
| This journal is © The Royal Society of Chemistry 2014 |





