Branched platinum–acetylide complexes: synthesis, properties, and their aggregation behavior†
Jing Zhanga,
Nai-Wei Wua,
Xing-Dong Xua,
Quan-Jie Lib,
Cui-Hong Wanga,
Hongwei Tanb and
Lin Xu*a
aDepartment of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062, People's Republic of China. E-mail: lxu@chem.ecnu.edu.cn
bDepartment of Chemistry, Beijing Normal University, Beijing 100050, People's Republic of China
First published on 24th March 2014
Abstract
A new branched platinum–acetylide complex PP6 containing pyrene as the main skeleton, pentiptycene units as bridges, and long alkyl chains as branches was successfully synthesized. The structure of PP6 was well characterized by 1H NMR, 31P {1H} NMR, MALDI-TOF-MS spectrometry, and the semiempirical PM6 method. The investigation of the absorption and emission spectra of PP6 and the model complexes revealed that the introduction of iptycene was beneficial to improve the emission efficiency. More importantly, PP6 was aggregated into ordered microspheres driven by the hydrophobic/hydrophilic interactions. The morphologies of the microspheres were investigated by SEM, TEM, LSCM, and EDX. Furthermore, the morphologies and the sizes of the microscale aggregates could be changed by altering the iptycene moiety or the hydrophobic units in PP6.
Introduction
During the past few decades, the design and self-assembly of ordered micro/nanostructures from small molecular building blocks via a “bottom-up” strategy have attracted tremendous attention owing to their wide applications in supramolecular chemistry and materials science.1 To date, many different types of small molecules have been employed to construct micro/nano architectures bearing various functionalities.2 Among these small molecules, organic conjugated molecules have evolved to be one of the most widely investigated building blocks due to their diverse applications in the fields of chemical sensors, solar cells, semiconductors, liquid crystals, optical storage devices, laser dyes, electrochromic displaying systems, and non-linear optics.3 As a π-conjugated molecule, pyrene and its derivatives have been widely explored in the field of luminescent material.4 With the introduction of ethynyl subunits into the pyrene skeleton, the emission characteristics of pyrene species can be bathochromatically tuned to the visible region by extending the π-conjugated systems.5 However, up to now, there have been few examples on preparation ordered micro/nanostructures with 1,3,6,8-tetraethynyl substituted pyrene as skeleton.6It is well known that the precise control over the micro/nano aggregation morphologies in a long-range order is at the centre stage of nanoscience and technology because the morphology of the functional material plays an essential role in determining the functionality of the final devices.7 There are many factors, such as the chemical structure of the building blocks,8 solvent,9 and temperature,10 will influence the formation of aggregation morphologies. Currently, there has been great interest in investigating the influence of molecular structural effect (e.g., steric hindrance) on the formation of aggregate morphology. However, the study of steric hindrance on aggregation is still at the early stage.11 Thus, it is still necessary to investigate the influence of steric hindrance on the morphology of aggregation.
Iptycene is an interesting class of structurally unique compounds consisting of more than three arene units fused together through a [2.2.2] bicyclooctatriene framework.12 Triptycene and pentiptycene are the most common members of iptycene. Because of their unique geometrical, structural, and electronic characteristics, their derivatives have received significant attention and been applied in a wide range of fields, such as chemical sensors, charge transfer, conjugated polymeric materials, molecular machinery, gas absorption/storage, etc.13 Notably, it has been reported that the incorporation of rigid three-dimensional iptycene moieties into conjugated polymer backbones would prevent π-stacking of the polymer backbones and thereby maintain high fluorescence quantum yields and stable spectroscopic behavior.14 For instance, Swager's group14a has designed and synthesized the pentiptycene-containing poly (phenylene ethynylene) (PPE), which had high fluorescence quantum yield and reduced Stokes shift caused by the rigid and planar conformation of pentiptycene-containing PPE in LCs. Thus, we anticipated that the introduction of different iptycene into the platinum–acetylide skeleton would provide a platform to gain an insight into the steric hindrance effect on both optical and aggregate morphology property.
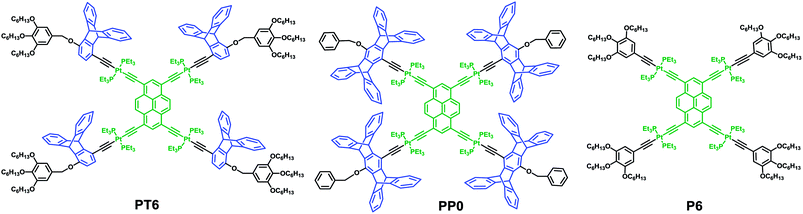
It is worthy to be noted that both pentiptycene and pyrene, which consist of multiple fused benzene rings, have widely applications in the fields of supramolecular chemistry and materials sciences, respectively.12,15 The introduction of iptycene onto the peripheral positions of pyrene may prevent self-aggregation and expand conjugation of pyrene, thereby generate a new series of complexes with interesting properties such as high fluorescence quantum yield and long-wavelength emission. Based on the fact that iptycene is a steric hindrance group and π-conjugated molecule and previous works reported by our group on platinum–acetylide chemistry,16–18 we envisioned that the introduction of iptycene moiety into the skeleton might alter the optical and aggregation properties of platinum–acetylide complexes such as high fluorescence quantum yields, long-wavelength emission, and stable spectroscopic behavior. Herein, the new branched platinum–acetylide molecule PP6 (Fig. 1) was designed that included the following merits: (i) 1,3,6,8-tetraethynylpyrene skeleton was employed as the centre core, which allowed that the absorption and emission peaks shifted to longer wavelength.19 (ii) Platinum–acetylide units were used as bridges, which may lead to the versatile spectroscopic properties.20 (iii) Four pentiptycene fragments were introduced to the periphery of pyrene units to obtain the steric hindrance effect on optical and aggregation property. (iv) Long alkyl chains were decorated at the periphery of PP6, which enabled the orientation and ordered aggregation of the molecules via hydrophobic interaction.21 The optical properties and aggregation behavior of PP6 have been investigated.
Experiments
General information
All solvents were dried according to standard procedures and all of them were degassed under N2 for 30 minutes before use. Reagents were used as purchased. All air-sensitive reactions were carried out under nitrogen atmosphere. 1H NMR, 13C NMR, and 31P NMR spectra were recorded on Bruker 400 MHz Spectrometer (1H: 400 MHz; 13C: 100 MHz; 31P: 161.9 MHz) at 298 K. The 1H and 13C NMR chemical shifts are reported relative to residual solvent signals, and 31P NMR resonances are referenced to an internal standard sample of 85% H3PO4 (δ 0.0). Coupling constants (J) are denoted in Hz and chemical shifts (δ) in ppm. Multiplicities are denoted as follows: s = singlet, d = doublet, m = multiplet, br = broad.MALDI mass spectroscopy analysis was performed on an IonSpec HiResMaldi Fourier Transform Mass Spectrometer (IonSpec Co., California, USA) using an accelerating potential of 30 V. UV-vis spectra were recorded on a Cary 50 Bio UV-vis Spectrophotometer. Emission spectra were measured on a Cary eclipse luminescence spectrometer. Samples for absorption and emission measurements were contained in 1 cm quartz cuvettes. SEM images were obtained using a S-4800 (Hitachi Ltd.) with an accelerating voltage of 3.0–10.0 kV. Samples were prepared by dropping solutions onto a silicon wafer. TEM images were recorded on a Tecnai G2 F30 (FEI Ltd.). The sample for TEM measurement was prepared by dropping the solution onto a carbon-coated copper grid. EDX measurements were performed using a S-4800 (Hitachi Ltd.) with an voltage of 10.0 kV. LSCM imaging was performed with an OLYMPUS ZX81 laser scanning microscopy, 362 nm laser was selected as the excitation. Semi-empirical PM6 method was used to optimize the geometry and calculate the final energy. The solvation effects were estimated by using the conductor-like polarizable continuum model (CPCM). Dielectric constant of 8.93 was used for CH2Cl2 solution. All calculations have been carried out with Gaussian 09.
Synthesis of complex PP6
Under an atmosphere of nitrogen, 5 mL THF and 5 mL Et2NH were added to a mixture of compound 4 (120 mg, 0.048 mmol), compound 3 (327 mg, 0.380 mmol), and CuI (2.5 mg, 0.013 mmol). The mixture was then stirred at room temperature for 3 hours. Column chromatography with dichloromethane–petroleum (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent afforded the yellow solid of PP6 with a yield of 82.1% (212 mg); Rf = 0.42 (dichloromethane–petroleum ether 2
1) as eluent afforded the yellow solid of PP6 with a yield of 82.1% (212 mg); Rf = 0.42 (dichloromethane–petroleum ether 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Mp: 165 °C. 1H NMR (CDCl3, 400 MHz): δ 8.77 (s, 4H), 8.13 (s, 2H), 7.30–7.28 (m, 16H), 7.24–7.22 (m, 16H), 6.92 (br, 32H), 6.85 (s, 8H), 6.04 (s, 8H), 5.65 (s, 8H), 4.88 (s, 8H), 4.12–4.06 (m, 24H), 2.43 (br, 48H), 1.92–1.82 (m, 24H), 1.50–1.39 (m, 144H), 0.92 (br, 36H). 13C NMR (CDCl3, 100 MHz): δ 153.49, 147.48, 146.02, 145.51, 143.98, 138.26, 134.22, 132.94, 132.41, 130.03, 125.36, 124.91, 123.45, 123.36, 116.87, 114.04, 112.94, 109.42, 106.40, 104.04, 77.68, 73.54, 69.37, 52.28, 48.51, 37.79, 31.60, 30.35, 29.64, 29.46, 25.81, 22.68, 22.62, 16.78, 14.07, 14.01, 8.78. 31P NMR (CDCl3, 161.9 Hz): δ 12.88 (s, 1JPt–P = 2371.8 Hz). MALDI-TOF-MS of PP6, m/z calcd for C316H378O16P8Pt4, 5459.53 (M+), found 5459.22.
1). Mp: 165 °C. 1H NMR (CDCl3, 400 MHz): δ 8.77 (s, 4H), 8.13 (s, 2H), 7.30–7.28 (m, 16H), 7.24–7.22 (m, 16H), 6.92 (br, 32H), 6.85 (s, 8H), 6.04 (s, 8H), 5.65 (s, 8H), 4.88 (s, 8H), 4.12–4.06 (m, 24H), 2.43 (br, 48H), 1.92–1.82 (m, 24H), 1.50–1.39 (m, 144H), 0.92 (br, 36H). 13C NMR (CDCl3, 100 MHz): δ 153.49, 147.48, 146.02, 145.51, 143.98, 138.26, 134.22, 132.94, 132.41, 130.03, 125.36, 124.91, 123.45, 123.36, 116.87, 114.04, 112.94, 109.42, 106.40, 104.04, 77.68, 73.54, 69.37, 52.28, 48.51, 37.79, 31.60, 30.35, 29.64, 29.46, 25.81, 22.68, 22.62, 16.78, 14.07, 14.01, 8.78. 31P NMR (CDCl3, 161.9 Hz): δ 12.88 (s, 1JPt–P = 2371.8 Hz). MALDI-TOF-MS of PP6, m/z calcd for C316H378O16P8Pt4, 5459.53 (M+), found 5459.22.
Synthesis of complex PP0
Under an atmosphere of nitrogen, 5 mL THF and 5 mL Et2NH were added to a mixture of compound 4 (120 mg, 0.047 mmol), compound 6 (160 mg, 0.285 mmol), and CuI (1.8 mg, 0.0095 mmol). The mixture was then stirred at room temperature for 5.5 hours. During this period yellow solid was precipitate. The resulted solid was filtered and washed with THF and water. The resulted yellow solid was collected with a yield of 90% (182 mg). Rf = 0.53 (dichloromethane–petroleum ether 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Mp: 248 °C. 1H NMR (CDCl3, 400 MHz): δ 8.77 (s, 4H), 8.13 (s, 2H), 7.73 (d, J = 7.2 Hz, 8H), 7.60–7.57 (m, 8H), 7.52–7.49 (m, 4H), 7.28 (br, 32H), 6.93 (br, 32H), 6.05 (s, 8H), 5.69 (s, 8H), 4.92 (s, 8H), 2.43 (br, 48H), 1.52–1.44 (m, 72H). 13C NMR (CDCl3, 100 MHz): δ 147.24, 146.06, 145.53, 143.98, 137.79, 134.19, 132.42, 130.06, 128.82, 128.25, 127.82, 125.42, 124.92, 123.46, 123.38, 123.10, 116.97, 113.99, 113.03, 109.45, 104.01, 77.56, 52.29, 48.42, 16.76, 8.76. 31P NMR (CDCl3, 161.9 Hz): δ 12.69 (s, 1JPt–P = 2373.5 Hz). MALDI-TOF-MS of PP0, m/z calcd for C244H234O4P8Pt4, 4258.47 (M+), found 4258.29.
1). Mp: 248 °C. 1H NMR (CDCl3, 400 MHz): δ 8.77 (s, 4H), 8.13 (s, 2H), 7.73 (d, J = 7.2 Hz, 8H), 7.60–7.57 (m, 8H), 7.52–7.49 (m, 4H), 7.28 (br, 32H), 6.93 (br, 32H), 6.05 (s, 8H), 5.69 (s, 8H), 4.92 (s, 8H), 2.43 (br, 48H), 1.52–1.44 (m, 72H). 13C NMR (CDCl3, 100 MHz): δ 147.24, 146.06, 145.53, 143.98, 137.79, 134.19, 132.42, 130.06, 128.82, 128.25, 127.82, 125.42, 124.92, 123.46, 123.38, 123.10, 116.97, 113.99, 113.03, 109.45, 104.01, 77.56, 52.29, 48.42, 16.76, 8.76. 31P NMR (CDCl3, 161.9 Hz): δ 12.69 (s, 1JPt–P = 2373.5 Hz). MALDI-TOF-MS of PP0, m/z calcd for C244H234O4P8Pt4, 4258.47 (M+), found 4258.29.
Synthesis of complex PT6
Under an atmosphere of nitrogen, 6.5 mL THF and 6.5 mL Et2NH were added to a mixture of compound 4 (160 mg, 0.063 mmol), compound 8 (260 mg, 0.38 mmol), and CuI (4.8 mg, 0.025 mmol). The mixture was then stirred at room temperature for 5 hours. Column chromatography with dichloromethane–petroleum (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent afforded the orange solid of PT6 with a yield of 68.2% (205 mg). Rf = 0.61 (dichloromethane). Mp: 99 °C. 1H NMR (CDCl3, 400 MHz): δ 8.63 (s, 4H), 7.99 (br, 2H), 7.38–7.36 (m, 16H), 6.98–6.94 (m, 20H), 6.65 (s, 8H), 6.51 (d, J = 8.4 Hz, 4H), 6.09 (s, 4H), 5.94 (s, 4H), 4.96 (s, 8H), 4.06–3.97 (m, 24H), 2.36 (br, 48H), 1.84–1.78 (m, 24H), 1.50–1.35 (m, 144H), 0.91 (br, 36H). 13C NMR (CDCl3, 100 MHz): δ 153.27, 151.46, 147.10, 146.03, 145.76, 137.90, 133.34, 132.60, 132.02, 129.98, 128.71, 125.31, 124.91, 124.88, 123.61, 123.04, 117.83, 114.08, 110.18, 109.13, 108.44, 106.57, 105.93, 73.42, 71.01, 69.15, 52.34, 47.44, 31.78, 31.59, 30.33, 29.40, 25.78, 22.69, 22.63, 16.69, 14.08, 14.02, 8.56. 31P NMR (CDCl3, 161.9 Hz): δ 12.57 (s, 1JPt–P = 2383.2 Hz). MALDI-TOF-MS of PT6, m/z calcd for C260H346O16P8Pt4, 4755.28 (M+), found 4755.42.
1) as eluent afforded the orange solid of PT6 with a yield of 68.2% (205 mg). Rf = 0.61 (dichloromethane). Mp: 99 °C. 1H NMR (CDCl3, 400 MHz): δ 8.63 (s, 4H), 7.99 (br, 2H), 7.38–7.36 (m, 16H), 6.98–6.94 (m, 20H), 6.65 (s, 8H), 6.51 (d, J = 8.4 Hz, 4H), 6.09 (s, 4H), 5.94 (s, 4H), 4.96 (s, 8H), 4.06–3.97 (m, 24H), 2.36 (br, 48H), 1.84–1.78 (m, 24H), 1.50–1.35 (m, 144H), 0.91 (br, 36H). 13C NMR (CDCl3, 100 MHz): δ 153.27, 151.46, 147.10, 146.03, 145.76, 137.90, 133.34, 132.60, 132.02, 129.98, 128.71, 125.31, 124.91, 124.88, 123.61, 123.04, 117.83, 114.08, 110.18, 109.13, 108.44, 106.57, 105.93, 73.42, 71.01, 69.15, 52.34, 47.44, 31.78, 31.59, 30.33, 29.40, 25.78, 22.69, 22.63, 16.69, 14.08, 14.02, 8.56. 31P NMR (CDCl3, 161.9 Hz): δ 12.57 (s, 1JPt–P = 2383.2 Hz). MALDI-TOF-MS of PT6, m/z calcd for C260H346O16P8Pt4, 4755.28 (M+), found 4755.42.
Results and discussion
PP6 was synthesized following the procedure as outlined in Scheme 1. The key precursors 1, 2, and 4 were prepared according to the procedures in the previous literatures.6b,22 The intermediate pentiptycene derivative 3 was prepared via the etherification reaction of compound 1 with benzyl bromide derivative 2 under a conventional SN2 reaction condition, followed by deprotection of the TMS group under a basic condition. Subsequent cross-coupling reaction of intermediate 3 with 4 in the presence of CuI resulted in the target complex PP6 in good yield (82.1%).The molecular structure of PP6 was characterized by multiple nuclear NMR (1H, 31P, and 13C) and mass spectrometry (MALDI-TOF-MS). The multinuclear NMR analysis of PP6 suggested the formation of discrete, well-defined, and highly symmetric structure. The 1H NMR analysis exhibited characteristic 1,3,6,8-tetrakis substituted pyrene proton resonances in the aromatic area (Fig. S6†). Two singlets at 8.77 ppm and 8.13 ppm were observed in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
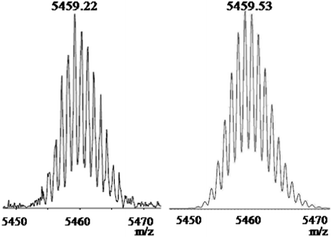
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for the typical protons on pyrene ring. Two singlets at 6.04 ppm and 5.65 ppm were assigned to the bridgehead protons of the pentiptycene units. The 31P {1H} NMR spectrum of PP6 displayed a sharp singlet (ca. 12.9 ppm) shifted downfield from the precursor 4 by approximately 3.5 ppm due to the electron withdrawing of the large π-conjugated bridges (Fig. S6†). This change, as well as the increase in coupling of flanking 195Pt satellites (ca. Δ1JPt–P = 48 Hz), is consistent with electron back-donation from the platinum atoms. In addition, the MALDI-TOF-MS spectrometric study of complex PP6 provided further strong support for the formation of the desired complex (Fig. 2). The MALDI-TOF-MS spectrum of PP6 exhibited a strong signal at 5459.22 Da, corresponding to the [M]+ species with a deviation of less than 0.31 Da. This peak was isotopically resolved and its isotopic resolution is in excellent agreement with the theoretical distribution. Therefore, all the above results supported the formation of desired pentiptycene-based 1,3,6,8-tetrakis substituted pyrene derivative.
1 ratio for the typical protons on pyrene ring. Two singlets at 6.04 ppm and 5.65 ppm were assigned to the bridgehead protons of the pentiptycene units. The 31P {1H} NMR spectrum of PP6 displayed a sharp singlet (ca. 12.9 ppm) shifted downfield from the precursor 4 by approximately 3.5 ppm due to the electron withdrawing of the large π-conjugated bridges (Fig. S6†). This change, as well as the increase in coupling of flanking 195Pt satellites (ca. Δ1JPt–P = 48 Hz), is consistent with electron back-donation from the platinum atoms. In addition, the MALDI-TOF-MS spectrometric study of complex PP6 provided further strong support for the formation of the desired complex (Fig. 2). The MALDI-TOF-MS spectrum of PP6 exhibited a strong signal at 5459.22 Da, corresponding to the [M]+ species with a deviation of less than 0.31 Da. This peak was isotopically resolved and its isotopic resolution is in excellent agreement with the theoretical distribution. Therefore, all the above results supported the formation of desired pentiptycene-based 1,3,6,8-tetrakis substituted pyrene derivative.
All attempts to obtain the crystal structure of PP6 have proven unsuccessful up to date. Therefore, in order to gain more structural information of PP6, semi-empirical PM6 method was employed to optimize the geometry of PP6 (Fig. 3). The optimized structure of PP6 featured a roughly rectangular plane geometry configuration with slight tetrahedral distortions. The width of the rectangle was 4.4 nm and the length was 4.8 nm. Moreover, the four substituted groups outside the central pyrene featured the same length of 2.7 nm.
As a π-conjugated molecule, pyrene and its derivatives possess rich optical properties. Therefore, the investigation of UV/visible absorption and emission spectroscopy of PP6 in dilute dichloromethane solution was firstly performed at 298 K. The photophysical data of PP6 were summarized in Table 1. The absorption spectrum of PP6 exhibited intense absorption band in the range of 450–525 nm, 325–400 nm, and 250–300 nm (Fig. 4). With the references to previous spectroscopic investigation on trans-[Pt(PEt3)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)2] and its derivatives,23 the low energy absorption bands are described as an admixture of intraligand (IL) [π–π* (C
CR)2] and its derivatives,23 the low energy absorption bands are described as an admixture of intraligand (IL) [π–π* (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)] and metal-to-ligand charge transfer (MLCT) [d(Pt) π–π* (C
CR)] and metal-to-ligand charge transfer (MLCT) [d(Pt) π–π* (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)] transition with predominant IL character. The higher energy absorption bands are likely due to π–π* and n–π* transitions localized on the alkoxyphenyl and pentiptycene fragments. It should be noted that the absorption spectrum of PP6 exhibited a red-shift of ∼156 nm compared to a naked pyrene (ca. 335 nm),24 which is attributed to the introduction of multiple conjugated ethynyl-phenyl fragments.
CR)] transition with predominant IL character. The higher energy absorption bands are likely due to π–π* and n–π* transitions localized on the alkoxyphenyl and pentiptycene fragments. It should be noted that the absorption spectrum of PP6 exhibited a red-shift of ∼156 nm compared to a naked pyrene (ca. 335 nm),24 which is attributed to the introduction of multiple conjugated ethynyl-phenyl fragments.
| Solvent | λabs [nm] | ε [M−1 cm−1] | λem [nm] | Φema [%] | |
|---|---|---|---|---|---|
| a The fluorescence quantum yields of these compounds were measured at low concentrations (e.g. 0.125 μM for PP6). | |||||
| PP6 | CH2Cl2 | 491 | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
508 | 40.4 |
| (c = 1.25 × 10−5 M) | 459 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
535 | ||
| 362 | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||||
| 270 | 229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
||||
| PP0 | CH2Cl2 | 490 | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 344 |
505 | 48.1 |
| (c = 1.31 × 10−5 M) | 458 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 305 |
535 | ||
| 363 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
||||
| 271 | 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
||||
| PT6 | CH2Cl2 | 491 | 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 778 778 |
507 | 42.5 |
| (c = 1.26 × 10−5 M) | 458 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 810 |
537 | ||
| 367 | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905 905 |
||||
| 278 | 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317 317 |
||||
| P6 | CH2Cl2 | 490 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
507 | 35.7 |
| (c = 1.13 × 10−5 M) | 458 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
538 | ||
| 368 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
||||
| 232 | 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
||||
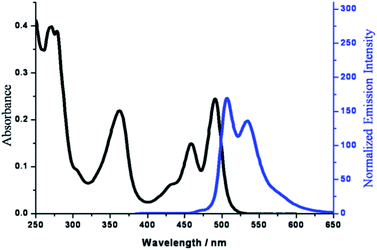 | ||
| Fig. 4 Absorption and emission spectra of PP6 in dilute dichloromethane solution at 298 K with a concentration of 0.1 μM (cuvette length = 0.2 cm). λex = 362 nm. | ||
Furthermore, the emission spectrum of PP6 in dilute dichloromethane solution was investigated as well. It is worth noting that PP6 exhibited strong luminescence upon UV irradiation (λex = 362 nm) in solution (Fig. 4). Compared to a naked pyrene (ca. 480 nm), PP6 displayed longer emission wavelength at λmax = 508 nm and 535 nm. Obviously, the introduction of conjugated ethynyl-phenyl fragments can efficiently tune the emission properties to the emission region with longer wavelength. Moreover, the mirror symmetry with the respect to the lowest energy absorption bands and the small Stokes shift of 17 nm was found in the emission spectrum of PP6, indicating the similar optical transition involving in both absorption and emission processes.17c
In order to explore the influence of pentiptycene to the optical properties, a series of model complexes PP0, PT6, and P6 with the similar skeleton as PP6, as shown in Scheme 2, were designed and synthesized as well. The model complexes PP0 and PT6 were prepared by using the similar procedure as described for the preparation of PP6. P6 was synthesized according to the previous procedures in the literature.6b Compared to PP6, in the case of PT6, pentiptycenes were replaced by triptycences.
Moreover, in order to investigate the steric hindrance effect on the optical and aggregation properties, P6 without iptycene moieties was synthesized. Furthermore, PP0 was prepared to study the influence of alky chains on the aggregate morphology. All these new complexes were confirmed by multiple nuclear NMR (1H, 31P, and 13C) and mass spectrometry. As shown in Table 1, it was found that PT6, PP0, and P6 exhibited the similar absorption and emission behavior compared to complex PP6. Moreover, the photoluminescence quantum yields of PP0, PP6, and PT6 (>40.0%) in dichloromethane were obtained by using quinine sulfate as reference,25 which were found to be higher than that of P6 (35.7%), indicating that the presence of iptycene was beneficial to improve the emission efficiency. This outcome might be attributed to the fact that the addition of steric hindrance group iptycene into the skeleton reduced the intramolecular interactions of the complexes, thus the intramolecular aggregation was blocked.
To gain a better understanding of the solvent influence on the optical property, solvent-dependent absorption and emission spectra of PP6 were recorded. Fig. S9 and S10 showed the normalized absorption and emission spectra of PP6 in toluene,† methylene chloride, tetrahydrofuran, chloroform, and acetonitrile. Both the absorption and emission spectra in all tested solvent were approximately mirror-imaged to one another, indicating a similar distribution of vibrational levels. As the solvent polarity increased (i.e. acetonitrile > tetrahydrofuran > chloroform > methylene chloride > toluene) the spectra profiles were almost constant, only in acetonitrile did the absorption and emission spectra of PP6 broadened. In polar solvent CH3CN, the solvent interacts with PP6 in its ground state or excited state, which induces the ground state and the excited state of PP6 to increase or decrease. Therefore, there are many different transition energies that become average together in the spectra of PP6, which causes peak-broadening. This outcome indicated that the vibrational levels of PP6 kept unchanged upon increasing the polarity of tested solvent.
With the aim to obtaining the aggregation behavior of PP6 in solution, further spectroscopic investigation of PP6 was carried out. Firstly, the investigation of concentration-variation spectroscopic spectra of PP6 was performed in dichloromethane. No significant change was observed in variable-concentration absorption of PP6. However, it was found that upon increasing the concentration of PP6 from 2.0 × 10−6 to 2.5 × 10−4 M at 298 K (Fig. 5), the intensity of the emission band at 505 nm decreased gradually, accompanying with an increase at 535 nm. Moreover, the emission peak at 535 nm became less structured and broader, which implied the formation of excimer of the pyrene chromophore at high concentration. It should be noted that, as shown in the inset of Fig. 5, upon UV irradiation at 365 nm, the emission color of PP6 changed from green to chartreuse upon increasing the concentration of PP6 from 1.0 × 10−5 to 1.0 × 10−4 M.
The addition of alky chains enabled PP6 to aggregate into the ordered microscale structure via hydrophobic interaction. Therefore, with the newly designed branched platinum–acetylide complexes in hand, the morphologies of these complexes were examined by scanning electronic microscopy (SEM), transmission electronic microscope (TEM), and laser scanning confocal microscopy (LSCM). The SEM, TEM, and LCSM samples were prepared by depositing the solution of complexes (toluene/n-propanol = 1/1, c = 0.25 mM) onto a SiO2/Si substrate (1 × 1 cm2), Cu grid or glass, respectively, then dried in air before morphology measurements. The SEM images of the branched complex PP6 recorded at different areas showed the presence of monodispersed microscale spheres with a diameter of 0.7–1.3 μm (Fig. 6). This spherical morphology was again proved by TEM, which displayed solid spherical architectures with the similar diameter as shown in SEM images. It is worth noting that the formation of spherical morphology was somewhat dependent to the polarity of the tested solvents. For example, changing the mixed solvents from toluene/n-propanol = 1/1 to hexane/dichloromethane = 1/1 or dichloromethane–methanol = 1/1, the spherical morphology was totally destroyed and the amorphous morphology was observed. It should be noted that microscale spherical architectures have attracted increasing interests during the past few decades because of their promising application such as photonic crystals, biosensors, and seed particles.26 Obviously, in this study, we present a simple yet efficient way to prepare microscale spherical aggregates from the branched platinum–acetylide complexes.
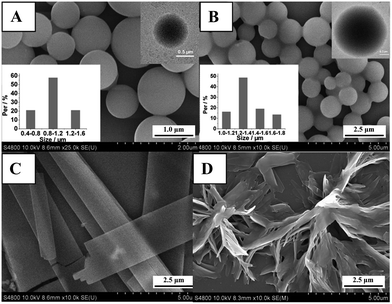 | ||
| Fig. 6 SEM images of PP6 (A), PT6 (B), PP0 (C) and P6 (D) in toluene/n-propanol = 1/1. Insert: TEM images. | ||
Further investigation revealed that the model complexes PP0, PT6, and P6 displayed entirely different morphologies. Compared to PP6, the aggregation morphology of PP0 was found to be micro-belts under the same condition (Fig. 6). This finding might be caused by the increase of the molecular polarity upon the removal of long alkyl chain, thus leading to the change of the aggregation behavior in the same solvent. Furthermore, model complex PT6 formed the similar microspheres but with doubled decreasing the steric hindrance by removing the iptycene moiety, complex P6 displayed fibrous morphology (Fig. 6) in the mixed solvents (toluene/n-propanol = 1/1). It should be noted that P6 can form luminescent gel in apolar solvent, such as cyclohexane, and the xerogel featured short sticks morphology.6b All above results indicated that the introduction of alky chains into the platinum–acetylide complexes could certainly promote the molecular aggregate tendency by increasing hydrophobic interaction. Moreover, introducing iptycene fragments into the platinum–acetylide skeleton may change the steric hindrance of the molecule, thus the final morphologies of the platinum–acetylide were altered.
With the aim to gaining the further insight into the morphologies and optical properties of these new micro-scale structures, LSCM was performed on PP6, PP0, and PT6. Notably, the green luminescence were emitted from the micro-scale structures of these complexes, which were directly observed by LSCM images as shown in Fig. 7, S13 and S14,† respectively. Moreover, the investigation of LSCM on samples PP6, PP0, and PT6 offered the similar morphologies as that observed in the SEM studies. The micro-belts, and spherical structures with different diameters were found in LSCM images of PP0, PP6, and PT6, respectively. It is worthy to be noted that the emission profiles of these microstructures were consistent with their emission profiles in dichloromethane (the concentration of all complexes was 0.25 mM) (Fig. S15†). All the above results suggested that these microstructures were entirely made of these branched complexes. Moreover, energy dispersive X-ray spectroscope (EDX) was used to obtain the elemental composition of these microstructures. For example, in the case of PP6 (Fig. 8), elemental composition of carbon, oxygen, platinum, and phosphorus were observed for the microspherical aggregates, which strongly supported that these microstructures were formed by PP6.
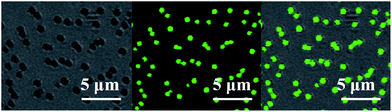 | ||
| Fig. 7 LCSM images of PP6 in toluene/n-propanol = 1/1. (a) Bright-field images, (b) fluorescence images, (c) the overlay of (a) and (b) (λex = 362 nm, emission was collected at 450–690 nm). | ||
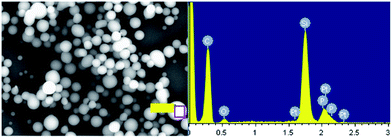 | ||
| Fig. 8 The EDX results of PP6 are collected from the area marked by the pink boxes shown at the right side of the image. | ||
Conclusions
In summary, we have successfully synthesized a new branched platinum–acetylide complex PP6 comprising a pyrene as main skeleton, four pentiptycene units as bridges, and alky chains as branch. The structure of PP6 was well characterized by 1H NMR, 31P {1H} NMR, MALDI-TOF-MS spectrometry, and semi-empirical PM6 method. PP6 showed versatile luminescence properties in solution. Caused by the reduction of intramolecular interactions by pentiptycene moiety, the photoluminescence quantum yield of PP6 was improved as compared to P6 without iptycene moiety. Further investigation indicated that the absorption and emission spectra of PP6 have little response to solvent polarity. The increase of the concentration of PP6 significantly altered the emission spectrum profile due to the excimer formation in high concentration.More importantly, driven by the hydrophobic/hydrophilic interaction, it was found that PP6 could aggregate into ordered microspheres. The microsphere was well characterized by using SEM, TEM, LSCM, and EDX. Interestingly, the morphologies and the sizes of the resulted aggregates could be modulated by changing the pentiptycene or hydrophobic units in PP6, which provided a simple yet efficient method for the generation of various nanostructures from branched platinum–acetylide complexes. These findings clearly enriched the library of branched platinum–acetylide complexes and provided a new platform to design potential functional micro/scale materials.
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (no. 21302058), the Fundamental Research Funds for the Central Universities, and the Research Fund for the Doctoral Program of Higher Education of China (no. 20130076120006). We also appreciate valuable discussion with Prof. Hai-Bo Yang (East China Normal University).Notes and references
- (a) M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960 CrossRef CAS PubMed; (b) L. Maggini and D. Bonifazi, Chem. Soc. Rev., 2012, 41, 211 RSC; (c) A. Ciesielski, C. A. Palma, M. Bonini and P. Samorì, Adv. Mater., 2010, 22, 3506 CrossRef CAS PubMed; (d) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766–1771 CrossRef CAS PubMed; (e) W. Li and T. Aida, Chem. Rev., 2009, 109, 6047 CrossRef CAS PubMed; (f) Y. Yan and J. Huang, Coord. Chem. Rev., 2010, 254, 1072 CrossRef CAS; (g) D.-L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC; (h) A. Deshpande, C.-H. Sham, J. M. P. Alaboson, J. M. Mullin, G. C. Schatz and M. C. Hersam, J. Am. Chem. Soc., 2012, 134, 16759 CrossRef CAS PubMed; (i) J. Wang and M. Jiang, J. Am. Chem. Soc., 2006, 128, 3703 CrossRef CAS PubMed; (j) K. Liu, Y. Yao, Y. Liu, C. Wang, Z. Li and X. Zhang, Langmuir, 2012, 28, 10697 CrossRef CAS PubMed; (k) Y. Ding, Y. Yang, L. Yang, Y. Yan, J. Huang and M. A. C. Stuart, ACS Nano, 2012, 6, 1004 CrossRef CAS PubMedY. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712 CrossRef CAS PubMed.
- (a) A. L. Kanibolotsky, I. F. Perepichka and P. J. Skabara, Chem. Soc. Rev., 2010, 39, 2695 RSC; (b) P. Dutta, W. Yang, W.-H. Lee, I. N. Kang and S.-H. Lee, J. Mater. Chem., 2012, 22, 10840 RSC; (c) L. Sun, F.-Q. Bai, Z.-X. Zhao and H.-X. Zhang, Sol. Energy Mater. Sol. Cells, 2011, 95, 1800 CrossRef CAS; (d) T. Lei, H.-B. Chen, J. Yin, S. Huang, X. Zhu and J. Pei, Org. Electron., 2011, 12, 453 CrossRef CAS; (e) K. H. Jung, S. Y. Bae, K. H. Kim, M. J. Cho, K. Lee, Z. H. Kim, D. H. Choi, D. H. Lee, D. S. Chung and C. E. Park, Chem. Commun., 2009, 5290 RSC; (f) H. Shang, H. Fan, Y. Liu, W. Hu, Y. Li and X. Zhan, J. Mater. Chem., 2011, 21, 9667 RSC; (g) L. Cai, R. Zhan, K.-Y. Pu, X. Qi, H. Zhang, W. Huang and B. Liu, Anal. Chem., 2011, 83, 7849 CrossRef CAS PubMed; (h) Z.-Q. Li, Y.-M. Zhang, H.-Z. Chen, J. Zhao and Y. Liu, J. Org. Chem., 2013, 78, 5110 CrossRef CAS PubMed; (i) K.-D. Zhang, T.-Y. Zhou, X. Zhao, X.-K. Jiang and Z.-T. Li, Langmuir, 2012, 28, 14839 CrossRef CAS PubMed; (j) T. Nakamura, H. Ube, M. Shiro and M. Shionoya, Angew. Chem., Int. Ed., 2013, 52, 720 CrossRef CAS PubMed; (k) K. Kondo, A. Suzuki, M. Akita and M. Yoshizawa, Angew. Chem., Int. Ed., 2013, 52, 2308 CAS.
- (a) L. Zhang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596 CrossRef PubMed; (b) N. Chandrasekhar and R. Chandrasekar, Chem. Commun., 2010, 46, 2915 RSC; (c) Z. B. Henson, K. Müllen and G. C. Bazan, Nat. Chem., 2012, 4, 699 CrossRef CAS PubMed; (d) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS PubMed; (e) J. F. Mike, A. J. Makowski and M. Jeffries-EL, Org. Lett., 2008, 10, 4915 CrossRef CAS PubMed.
- (a) T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260 CrossRef CAS PubMed; (b) G. He, N. Yan, J. Yang, H. Wang, L. Ding, S. Yin and Y. Fang, Macromolecules, 2011, 44, 4759 CrossRef CAS; (c) Y. Kamikawa and T. Kato, Langmuir, 2007, 23, 274 CrossRef CAS PubMed; (d) M. Ma, Y. Kuang, Y. Gao, Y. Zhang, P. Gao and B. Xu, J. Am. Chem. Soc., 2010, 132, 2719 CrossRef CAS PubMed; (e) B. Adhikari, J. Nanda and A. Banerjee, Chem. –Eur. J., 2011, 17, 11488 CrossRef CAS PubMed; (f) J. R. Moffat and D. K. Smith, Chem. Commun., 2011, 11864 RSC.
- (a) H. Maeda, T. Maeda, K. Mizuno and K. Fujimoto, Chem. –Eur. J., 2006, 12, 824 CrossRef CAS PubMed; (b) H. M. Kim, Y. O. Lee, C. S. Lim, J. S. Kim and B. R. Cho, J. Org. Chem., 2008, 73, 5127 CrossRef CAS PubMed; (c) S. Bernhardt, M. Kastler, V. Enkelmann, M. Baumgarten and K. Müllen, Chem. –Eur. J., 2006, 12, 6117 CrossRef CAS PubMed.
- (a) S. Diring, F. Camerel, B. Donnio, T. Dintzer, S. Toffanin, R. Capelli, M. Muccini and R. Ziessel, J. Am. Chem. Soc., 2009, 131, 18177 CrossRef CAS PubMed; (b) X.-D. Xu, J. Zhang, X. Yu, L.-J. Chen, D. X. Wang, T. Yi, F. Li and H.-B. Yang, Chem. –Eur. J., 2012, 18, 16000 CrossRef CAS PubMed.
- (a) D. Ke, C. Zhan, A. D. Q. Li and J. Yao, Angew. Chem., Int. Ed., 2011, 50, 3715 CrossRef CAS PubMed; (b) G. De Luca, A. Liscio, P. Maccagnani, F. Nolde, V. Palermo, K. Müllen and P. Samorì, Adv. Funct. Mater., 2007, 17, 3791 CrossRef CAS.
- L.-J. Chen, J. Zhang, J. He, X.-D. Xu, N.-W. Wu, D.-X. Wang, Z. Abliz and H.-B. Yang, Organometallics, 2011, 30, 5590 CrossRef CAS.
- (a) Y. Zhou, T. Yi, T. Li, Z. Zhou, F. Li, W. Huang and C. Huang, Chem. Mater., 2003, 15, 2974 Search PubMed; (b) W. Weng, Z. Li, A. M. Jamieson and S. J. Rowan, Macromolecules, 2009, 42, 236 CrossRef CAS; (c) J. Zhang, X.-D. Xu, L.-J. Chen, Q. Luo, N.-W. Wu, D.-X. Wang, X.-L. Zhao and H.-B. Yang, Organometallics, 2011, 30, 4032 CrossRef CAS.
- (a) M. Lescanne, A. Colin, O. Mondain-Monval, F. Fages and J.-L. Pozzo, Langmuir, 2003, 19, 2013 CrossRef CAS; (b) L. Li, R.-Y. Wang, X.-Y. Liu and H.-H. Pan, J. Phys. Chem. B, 2009, 113, 5011 CrossRef PubMed; (c) F. Rodriguez-Llansola, J. F. Miravet and B. Escuder, Chem. Commun., 2009, 209 RSC.
- (a) K. Miszta, J. de Graaf, G. Bertoni, D. Dorfs, R. Brescia, S. Marras, L. Ceseracciu, R. Cingolani, R. van Roij, M. Dijkstra and L. Manna, Nat. Mater., 2011, 10, 872 CrossRef CAS PubMed; (b) H. Choi, J. H. Ko, Y. H. Kim and S. Jeong, J. Am. Chem. Soc., 2013, 135, 5278 CrossRef CAS PubMed; (c) M. Grzelczak, A. Sánchez-Iglesias, H. H. Mezerji, S. Bals, J. Pérez-Juste and L. M. Liz-Marzán, Nano Lett., 2012, 12, 4380 CrossRef CAS PubMed; (d) N. A. Gothard, M. W. Mara, J. Huang, J. M. Szarko, B. Rolczynski, J. V. Lockard and L. X. Chen, J. Phys. Chem. A, 2012, 116, 1984 CrossRef CAS PubMed.
- (a) J. H. Chong and M. J. MacLachlan, Chem. Soc. Rev., 2009, 38, 3301 RSC; (b) T. M. Swager, Acc. Chem. Res., 2008, 41, 1181 CrossRef CAS PubMed.
- (a) D. K. Frantz, A. Linden, K. K. Baldridge and J. S. Siegel, J. Am. Chem. Soc., 2012, 134, 1528 CrossRef CAS PubMed; (b) Y. Jiang and C.-F. Chen, Eur. J. Org. Chem., 2011, 6377 CrossRef CAS; (c) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS; (d) J.-S. Yang, J.-L. Yan, C.-K. Lin, C.-Y. Chen, Z.-Y. Xie and C.-H. Chen, Angew. Chem., Int. Ed., 2009, 48, 9936 CrossRef CAS PubMed; (e) T. Xie, S.-Z. Hu and C.-F. Chen, J. Org. Chem., 2013, 78, 981 CrossRef CAS PubMed; (f) Y. Han, H.-Y. Lu, Q.-S. Zong, J.-B. Guo and C.-F. Chen, J. Org. Chem., 2012, 77, 2422 CrossRef CAS PubMed; (g) J.-B. Guo, Y. Jiang and C.-F. Chen, Org. Lett., 2010, 12, 5764 CrossRef CAS PubMed.
- (a) E. E. Nesterov, Z. G. Zhu and T. M. Swager, J. Am. Chem. Soc., 2005, 127, 10083 CrossRef CAS PubMed; (b) B. M. Polishak, S. Huang, J. Luo, Z. Shi, X. H. Zhou, A. Hsu and A. K. Y. Jen, Macromolecules, 2011, 44, 1261 CrossRef CAS.
- (a) H. J. Kim, J. Hong, A. Hong, S. Ham, J. H. Lee and J. S. Kim, Org. Lett., 2008, 10, 1963 CrossRef CAS PubMed; (b) T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260 CrossRef CAS PubMed; (c) P. Anzenbacher Jr, L. Mosca, M. A. Palacios, G. V. Zyryanov and P. Koutnik, Chem. –Eur. J., 2012, 18, 12712 CrossRef PubMed.
- (a) X.-D. Xu, H.-B. Yang, Y.-R. Zheng, K. Ghosh, M. M. Lyndon, D. C. Muddiman and P. J. Stang, J. Org. Chem., 2010, 75, 7373 CrossRef CAS PubMed; (b) N.-W. Wu, J. Zhang, D. Ciren, Q. Han, L. J. Chen, L. Xu and H.-B. Yang, Organometallics, 2013, 32, 2536 CrossRef CAS; (c) N.-W. Wu, Q.-J. Li, J. Zhang, J. He, J.-K. Ou-Yang, H. Tan, Z. Abliz and H.-B. Yang, Tetrahedron, 2013, 69, 5981 CrossRef CAS; (d) X.-D. Xu, J.-K. Ou-Yang, J. Zhang, Y.-Y. Zhang, H.-Y. Gong, Y. Yu and H.-B. Yang, Tetrahedron, 2013, 69, 1086 CrossRef CAS; (e) Q.-J. Li, G.-Z. Zhao, L.-J. Chen, H. Tan, C.-H. Wang, D.-X. Wang, D. A. Lehman, D. C. Muddiman and H.-B. Yang, Organometallics, 2012, 31, 7241 CrossRef CAS; (f) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596 CrossRef CAS PubMed; (g) Q. Han, L.-L. Wang, Q.-J. Li, G.-Z. Zhao, J. He, B. Hu, H. Tan, Z. Abliz, Y. Yu and H.-B. Yang, J. Org. Chem., 2012, 77, 3426 CrossRef CAS PubMed; (h) L.-J. Chen, Q.-J. Li, J. He, H. Tan, Z. R. Abliz and H.-B. Yang, J. Org. Chem., 2012, 77, 1148 CrossRef CAS PubMed; (i) G.-Z. Zhao, Q.-J. Li, L.-J. Chen, H. Tan, C.-H. Wang, D.-X. Wang and H.-B. Yang, Organometallics, 2011, 30, 5141 CrossRef CAS; (j) G.-Z. Zhao, Q.-J. Li, L.-J. Chen, H. Tan, C.-H. Wang, D. A. Lehman, D. C. Muddiman and H.-B. Yang, Organometallics, 2011, 30, 3637 CrossRef CAS; (k) X. Yan, B. Jiang, T. R. Cook, Y. Zhang, J. Li, Y. Yu, F. Huang, H.-B. Yang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 16813 CrossRef CAS PubMed; (l) L. Xu, L.-J. Chen and H.-B. Yang, Chem. Commun., 2014 10.1039/c3cc47484d.
- (a) X.-D. Xu, J. Zhang, L.-J. Chen, X.-L. Zhao, D.-X. Wang and H.-B. Yang, Chem. –Eur. J., 2012, 18, 1659 CrossRef CAS PubMed; (b) X.-D. Xu, J. Zhang, L.-J. Chen, R. Guo, D.-X. Wang and H.-B. Yang, Chem. Commun., 2012, 48, 11223 RSC; (c) B. Jiang, L.-J. Chen, L. Xu, S.-Y. Liu and H.-B. Yang, Chem. Commun., 2013, 49, 6977 RSC; (d) Z.-Y. Li, L. Xu, C.-H. Wang, X.-L. Zhao and H.-B. Yang, Chem. Commun., 2013, 49, 6194 RSC; (e) N.-W. Wu, J. Zhang, C.-H. Wang, L. Xu and H.-B. Yang, Monatsh. Chem., 2013, 144, 553 CrossRef CAS; (f) G.-Z. Zhao, L.-J. Chen, W. Wang, J. Zhang, G. Yang, D.-X. Wang, Y. Yu and H.-B. Yang, Chem. –Eur. J., 2013, 19, 10094 CrossRef CAS PubMed; (g) W. Wang and H.-B. Yang, Chem. Commun., 2014 10.1039/c3cc47485b.
- (a) W. Wu, J. Zhang, H.-B. Yang, B. Jin, Y. Hu, J. Hua, C. Jing, Y. Long and H. Tian, J. Mater. Chem., 2012, 22, 5382 RSC; (b) W. Wu, X. Xu, H.-B. Yang, J. Hua, X. Zhang, L. Zhang, Y. Long and H. Tian, J. Mater. Chem., 2011, 21, 10666 RSC; (c) Z.-Y. Li, W. Wu, Q. Zhang, B. Jin, J. Hua, H.-B. Yang and H. Tian, Chem. –Asian J., 2013, 8, 2660 CrossRef CAS PubMed.
- Z. Zhao, S. Ye, Y. Guo, Z. Chang, L. Lin, T. Jiang, J. W. Y. Lam, P. Lu, H. Qiu, Y. Liu and B. Z. Tang, Org. Electron., 2011, 12, 2236 CrossRef CAS.
- (a) K. Glusac, M. E. Köse, H. Jiang and K. S. Schanze, J. Phys. Chem. B, 2007, 111, 929 CrossRef CAS PubMed; (b) M. C. Davis, L. C. Baldwin and T. J. Groshens, Tetrahedron Lett., 2012, 53, 1564 CrossRef CAS; (c) S. Y.-L. Leung, A. Y.-Y. Tam, C.-H. Tao, H. S. Chow and V. W.-W. Yam, J. Am. Chem. Soc., 2012, 134, 1047 CrossRef CAS PubMed.
- (a) B. Liu, M. Yang, N. Xia, P. Zheng, W. Wang and C. Burger, Soft Matter, 2012, 8, 9545 RSC; (b) W. Tao, Y. Liu, B. Jiang, S. Yu, W. Huang, Y. Zhou and D. Yan, J. Am. Chem. Soc., 2012, 134, 762 CrossRef CAS PubMed; (c) A. Ajayaghosh and S. J. George, J. Am. Chem. Soc., 2001, 123, 5148 CrossRef CAS PubMed; (d) S. J. George, A. Ajayaghosh, P. Jonkheijm, A. P. H. J. Schenning and E. W. Meijer, Angew. Chem., Int. Ed., 2004, 43, 3422 CrossRef CAS PubMed; (e) K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834 CrossRef CAS PubMed.
- (a) J.-S. Yang, J.-L. Yan, C.-Y. Hwang, S.-Y. Chiou, K.-L. Liau, H.-H. G. Tsai, G.-H. Lee and S.-M. Peng, J. Am. Chem. Soc., 2006, 128, 14109 CrossRef CAS PubMed; (b) K. Tanabe, T. Yasuda, M. Yoshio and T. Kato, Org. Lett., 2007, 9, 4271 CrossRef CAS PubMed.
- (a) M. Hissler, A. Harriman, A. Khatyr and R. Ziessel, Chem. –Eur. J., 1999, 5, 3366 CrossRef CAS; (b) C. H. Tao, N. Zhu and V. W.-W. Yam, Chem. –Eur. J., 2005, 11, 1647 CrossRef CAS PubMed.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039 CrossRef CAS.
- N. J. Demas and G. A. Crosby, J. Am. Chem. Soc., 1970, 92, 7262 CrossRef.
- (a) Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Sessler, Y. Kim and J. S. Kim, Chem. –Eur. J., 2010, 16, 58895 Search PubMed; (b) S. Burattini, H. M. Colquhoun, B. W. Greenland, W. Hayes and M. Wade, Macromol. Rapid Commun., 2009, 30, 459 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46957c |
| This journal is © The Royal Society of Chemistry 2014 |