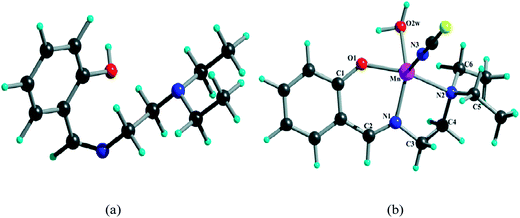
A light harvesting mononuclear manganese(II) complex: synthesis, characterization, DFT and TDDFT calculations and photophysical profile†
Debalina Ghosh,
Urmila Saha and
Kalyan K. Mukherjea*
Department of Chemistry, Jadavpur University, Calcutta (Kolkata), 700032, India. E-mail: k_mukherjea@yahoo.com; Fax: +91-33-2414-6584; Tel: +91-9831129321
First published on 14th March 2014
Abstract
A new manganese(II) [MnII(DEMP)(NCS)(H2O)] (DEMP = Schiff base derived from salicylaldehyde and 2-diethylaminoethylamine) complex has been synthesized and characterized by ESI-mass, IR, UV-vis, 1H and 13C NMR, EPR and thermogravimetric analysis. The structure of the complex has been optimized by DFT and TDDFT calculations. The complex has been found to exhibit strong fluorescence emission in different solvents of various polarities which is characterized by longer lifetimes in more polar solvents, accompanied by a reasonably good quantum yield. The complex is capable of absorbing light ranging from 200–850 nm. Thus, the molecule contains light harvesting components that can harvest the entire range of sunlight falling on earth.
Introduction
Manganese is an important metal in biological systems. It is essential for all forms of life. It accumulates in mitochondria where it is essentially required. Manganese plays an obligatory role in many cellular processes including lipid, protein and carbohydrate metabolism and is used as a cofactor by a diverse array of enzymes.1,2 The element is an essential trace mineral for all known living organisms. Manganese (Mn) is required for the growth and survival of most, if not all, living organisms. Until recently, relatively little was known about how bacteria take up trace nutrients such as manganese, nickel, copper and zinc, or how they regulate intracellular levels of these metal ions in response to availability and demand. Several metalloenzymes of manganese are known, such as arginase, pyruvate carboxylase and superoxide dismutase. Manganese enzymes are essential particularly in the detoxification of superoxide free radicals in micro-organisms3–9 and decomposition of O2− radical, using superoxide dismutase. For years together, it has been known that oxygen evolving photosynthetic organisms have a requirement for manganese.10,11 Depletion of manganese in plants or algae by withholding it from the growth medium leads to the loss of O2 evolution capability.12 Various experiments point to a site on the donor side of photosystem II as the location for the manganese.13–16 To date, it has not been possible to detect directly the Mn-containing entity that is responsible for mediating O2 evolution. It can be concluded that, the most primordial example of biological importance of manganese, is the photo-induced oxidation of water to molecular oxygen in photosystem II (PS II) of green plants.17–22 Manganese is believed to be an essential component, in this regard. The photo-activation of manganese complexes is now of interest not only for modelling of PS II, but also for the development of new photo active-materials.23–31 The photosynthetic manganese catalyzed reaction in PS II is solely dependent on the light harvesting process. Nature has deployed a number of pigments to harvest the entire range of sunlight falling on earth. Artificial photosynthesis is an area of active research which, as an integral part, requires light harvesting components. So, scientists are active to explore the possibility of developing artificial light harvesting compounds.32–38 The effective light harvesting compounds should have the ability to absorb light of a wide range of wavelengths to be as close as possible to the natural photosynthetic light harvesting pigments. In the present project, attempt has been made to develop a light harvesting mononuclear manganese complex. The complex has been synthesized and characterized spectroscopically. The structure of the complex has been optimized with theoretical DFT calculations and detailed photophysical properties have been examined for its exploitation as a possible light harvesting agent as it is capable of harvesting light of the entire visible region i.e. the entire region of the sunlight falling on earth.Results and discussion
Characterization of the ligand
Therefore the ligand can be formulated as C13H20N2O.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and OH group frequencies at around 1621 cm−1 and 3418 cm−1 respectively.
N) and OH group frequencies at around 1621 cm−1 and 3418 cm−1 respectively.Characterization of the complex
Therefore the molecular formula of the compound is MnC14H22N3O2S.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group40–43 in coordination, whereas the νOH band shifts to 3172 cm−1 suggesting the engagement of this group in coordination with the metal centre. The band at 3386 cm−1 in the complex arises due to the vibration of metal oxygen bond of the axially coordinated water molecule (νMn(1)–O(2)). The bands at 1598.8, 983.85, 1095.93 cm−1 are due to νMn–N(11), νMn–N(14) and νMn–O(3) vibrations respectively of the coordinated Schiff base (S2†). Infrared bands due to NCS groups, νCN 2054 cm−1, νCS 800 cm−1 are consistent with terminally N-bonded NCS groups in the complex.44,45
N– group40–43 in coordination, whereas the νOH band shifts to 3172 cm−1 suggesting the engagement of this group in coordination with the metal centre. The band at 3386 cm−1 in the complex arises due to the vibration of metal oxygen bond of the axially coordinated water molecule (νMn(1)–O(2)). The bands at 1598.8, 983.85, 1095.93 cm−1 are due to νMn–N(11), νMn–N(14) and νMn–O(3) vibrations respectively of the coordinated Schiff base (S2†). Infrared bands due to NCS groups, νCN 2054 cm−1, νCS 800 cm−1 are consistent with terminally N-bonded NCS groups in the complex.44,45| Solvent | Complex λmax (nm) 10−4 (M) | Ligand λmax (nm) 10−4 (M) |
|---|---|---|
| DMSO | 263 | 258 |
| DMF | 269 | 266 |
| Acetonitrile | 288 | 254 |
| MeOH | 254 | 253 |
| Acetone | 325 | 333 |
| DCM | 299 | 259 |
| THF | 289 | 269 |
| CHCl3 | 305 | 256 |
| Bond length (Å) | 6-31G | 6-311G | 6-31++G |
|---|---|---|---|
| Mn–N1 | 2.150 | 2.162 | 2.151 |
| Mn–N2 | 2.408 | 2.387 | 2.428 |
| Mn–N3 | 2.007 | 2.012 | 2.005 |
| Mn–O1 | 2.088 | 2.089 | 2.083 |
| Mn–O2w | 2.231 | 2.226 | 2.259 |
| Bond angles (°) | |||
| N1–Mn–N2 | 78.223 | 78.258 | 77.893 |
| N2–Mn–N3 | 101.560 | 96.239 | 101.489 |
| N3–Mn–O2w | 103.002 | 99.275 | 102.249 |
| O1–Mn–O2w | 69.379 | 70.533 | 69.910 |
| O1–Mn–N1 | 83.508 | 83.530 | 83.861 |
| O1–Mn–N2 | 141.408 | 150.703 | 140.391 |
| N1–Mn–N3 | 124.351 | 138.712 | 122.928 |
| N2–Mn–O2w | 98.996 | 100.157 | 99.077 |
| O1–Mn–N3 | 116.775 | 112.477 | 117.893 |
| N1–Mn–O2w | 132.336 | 122.010 | 134.574 |
| Bond lengths (Å) | ||
|---|---|---|
| Free ligand (DEMP) | Complex | |
| O1–C1 | 1.388 | 1.330 |
| N1–C2 | 1.277 | 1.307 |
| N1–C3 | 1.480 | 1.472 |
| N2–C4 | 1.479 | 1.492 |
| N2–C5 | 1.491 | 1.509 |
| N2–C6 | 1.481 | 1.501 |
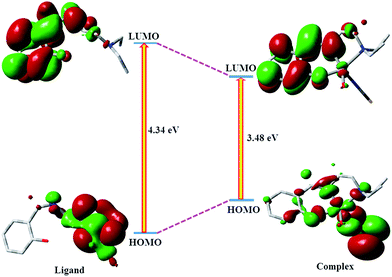
In case of DEMP at ground state, the electron density at HOMO−2, LUMO and LUMO+1 orbitals mainly resides on the benzene moiety while a considerable contribution comes from 2-diethylaminoethylamine moiety along with the contribution of benzene moiety in HOMO−1 and HOMO orbitals. The energy difference between HOMO and LUMO is 4.34 eV of ligand (DEMP) (Fig. 5). In case of the complex all the LUMO+2, LUMO+7and LUMO+8 orbitals are mainly originating from ligand π and π* orbital contributions while the HOMO and LUMO+1 orbitals arise from the contribution of metal d orbitals along with ligand π orbital. The energy difference between HOMO and LUMO is 3.48 eV in the complex (Fig. 5). These compositions are useful in understanding the nature of transition as well as the absorption spectra of both the ligand and complex (vide infra).
The ligand shows two absorption bands at 310 and 253 nm in methanolic solution at room temperature and both have ILCT character. This assignment was supported by TDDFT calculations. These two absorption bands can be assigned to the S0 → S1 and S0 → S6 (Fig. 6) transitions. The absorption energies along with their oscillator strengths, the main configurations and their assignments were calculated using TDDFT method and the related data are given in Table 4.
| Electronic transition | Composition | Excitation energy | Oscillator strength (f) | CI | Assign | λtheo (nm) | λexp (nm) |
|---|---|---|---|---|---|---|---|
| S0 → S1 | HOMO−2 → LUMO | 3.9974 eV (310 nm) | 0.1413 | 0.32108 | ILCT | 310 | 312 |
| HOMO → LUMO | 0.62216 | ILCT | |||||
| S0 → S6 | HOMO−2 → LUMO+1 | 4.8968 eV (253 nm) | 0.0851 | 4.8968 | ILCT | 253 | 253 |
| HOMO−1 → LUMO + 1 | 0.25357 | ILCT |
The complex shows two absorption bands at 375 and 315 nm in methanolic solution at room temperature. The absorptions calculated from TDDFT show bands at 379 and 333 nm for the complex (Fig. 4). This calculated value is in excellent agreement with the experimental results. These two absorption bands can be assigned to the S0 → S15 and S0 → S20 (Fig. 7) transitions, respectively and both the transitions originate from an admixture of MLCT and ILCT transitions (Table 5).
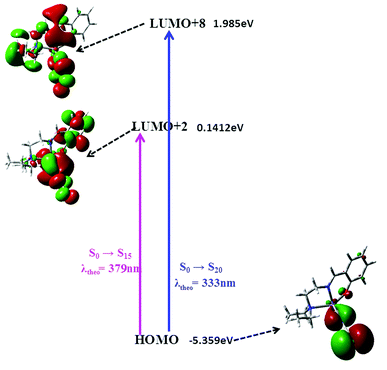 | ||
| Fig. 4 Frontier molecular orbitals involved in the UV-vis absorption of the metal complex calculated by TDDFT. | ||
| Electronic transition | Composition | Excitation energy | Oscillator strength (f) | CI | Transition assigned | λtheo (nm) | λexp (nm) |
|---|---|---|---|---|---|---|---|
| S0 → S15 | HOMO−2 → LUMO+5 | 3.2691 eV (379 nm) | 0.0106 | −0.11382 | ILCT | 379 | 375 |
| HOMO−3 → LUMO+5 | −0.14107 | ILCT | |||||
| HOMO → LUMO+2 | 0.89978 | MLCT/ILCT | |||||
| HOMO → LUMO+1 | −0.14828 | MLCT/ILCT | |||||
| S0 → S20 | HOMO → LUMO+7 | 3.7183 eV (333 nm) | 0.0527 | −0.23910 | MLCT/ILCT | 333 | 315 |
| HOMO → LUMO+8 | 0.95162 | MLCT/ILCT |
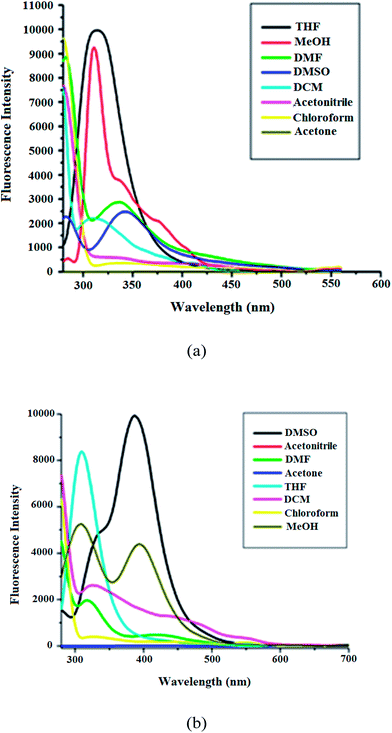
The fluorescence emission spectra of 10−4, 10−5 and 10−6 M ligand solutions of 2-[(2-diethylamino ethylimino)-methyl]-phenol impart different fluorescence intensities (S1(a)†) with same shape of spectra, and the effect of the polarity of solvent on the ligand also show similar patterns, except for THF, in which the emission spectrum does not split but show almost one sharp emission, with the highest fluorescence intensity (Fig. 8a).
The lowest polarity of the solvent may be the reason for this behaviour, therefore, the change in emission of the ligand might have occurred because of some sort of structural change in THF which dictates such behaviour. Hence, it can be concluded that the shape of the emission spectra depend neither on the concentration of the solutions, nor on the polarity of solvent. The emission spectra of solutions of the manganese(II) complex of 10−4, 10−5 and 10−6 M also exhibit same pattern as in the case of free ligand, when excited at 280 nm (S1(b)†). The complex has been found to exhibit strong fluorescence emission in different solvents of various polarities, compared to free Schiff base (Fig. 9a and b), which may be due to increased structural rigidity and for the enhanced metal–ligand interaction48,49 in the complex. The complex [MnII (DEMP) (NCS) (H2O)] exhibits efficient fluorescence emission with good quantum yield of Φf = 0.022 which was only 0.007 in free Schiff base. The quantum yields were determined using the equation:50
 | ||
| Fig. 9 (a) Emission spectra of ligand and complex at room temperature (λex = 280 nm) in DMSO (10−5 M). (b) Relative intensities of ligand and complex with respect to polarity of the solvents. | ||
From the lifetime measurements51 of both the ligand and the complex, it is also evident that the complex has shorter lifetime of fluorescence decay than the ligand and the lifetime increases with increasing polarity of the solvents (Fig. 10 and Table 6, a and b) both for the complex as well as for the ligand. Ligand exhibits higher lifetime in more polar solvents than the complex, so either there is a possibility of the involvement of the ligand in different excited state reactions like internal conversion and inter system crossings or the more polar excited state of the ligand get more stabilized in polar solvents and reside longer, exhibiting higher lifetime.
 | ||
| Fig. 10 Fluorescence lifetime spectra for (a) ligand and (b) complex in various solvents for 10−5 M at 280 nm excitation wavelength. | ||
| (a) | |||||
|---|---|---|---|---|---|
| Solvents | Lifetime (ns) | Amplitude | |||
| τ1 | τ2 | A1 | A2 | χ2 | |
| DMSO | 1.98 | 7.69 | 0.902 | 0.098 | 1.069 |
| DMF | 2.00 | 12.0 | 0.738 | 0.262 | 1.066 |
| Acetonitrile | 2.11 | 5.00 | 0.823 | 0.176 | 1.046 |
| MeOH | 1.654 | 2.530 | 0.7247 | 0.2753 | 1.13 |
| DCM | 0.93 | 6.94 | 0.834 | 0.166 | 1.105 |
| THF | 0.11 | 2.00 | 0.974 | 0.026 | 1.006 |
| Chloroform | 0.12 | 1.0 | 0.997 | 0.003 | 1.066 |
| (b) | |||||
|---|---|---|---|---|---|
| Solvents | Lifetime (ns) | Amplitude | |||
| τ1 | τ2 | A1 | A2 | χ2 | |
| DMSO | 5.248 | — | 1.303 | — | 1.212 |
| DMF | 1.061 | 3.124 | 0.930 | 0.068 | 1.209 |
| Acetonitrile | 2.199 | 5.72 | 0.950 | 0.141 | 1.103 |
| MeOH | 0.0758 | 1.764 | 0.980 | 0.011 | 1.028 |
| DCM | 0.435 | 2.692 | 0.950 | 0.050 | 1.125 |
| THF | 0.142 | 1.177 | 0.990 | 0.012 | 0.873 |
| Chloroform | 0.131 | 1.209 | 0.980 | 0.017 | 1.029 |
To observe the solvent effect, we calculated the Stokes shifts (Table 7.) and plotted them against the polarity of the solvents (Fig. 11). The Stokes' shift is seen to increase in most of the cases with an increase in the polarity of the solvent, which may be interpreted in accordance with an assumption that the complex exists in a less polar structure in less polar solvents and shows more polarity in higher polar solvents.52
| Solvents | Complex 10−4 M (nm) | Ligand 10−4 M (nm) | Complex 10−5 M (nm) | Ligand 10−4 M (nm) | Complex 10−6 M (nm) | Ligand 10−6 M (nm) |
|---|---|---|---|---|---|---|
| DMSO | 130 | 49 | 124 | 83 | 78 | 82 |
| DMF | 141 | 68 | 115 | 71 | 65 | 69 |
| Acetonitrile | 41 | 147 | 30 | 82 | 101 | 82 |
| MeOH | 157 | 49 | 55 | 48 | 46 | 48 |
| Acetone | 84 | 53 | 87 | 63 | 71 | 69 |
| DCM | 37 | 258 | 37 | 83 | 30 | 77 |
| THF | 37 | 44 | 21 | 40 | 21 | 69 |
| Chloroform | 27 | 185 | 22 | 84 | 27 | 80 |
Experimental
Materials
Salicylaldehyde, 2-diethylaminoethylamine, and hydroxylamine hydrochloride were obtained from Merck (India). Potassium permanganate was procured from Rankem and ammonium thiocyanate was obtained from SRL India. Solvents were purified by standard procedures,53 wherever necessary.Physical measurements
The IR spectra were taken as KBr discs at room temperature on a Perkin Elmer RFX-I IR spectrophotometer. UV-vis spectra (200–800 nm) were recorded at room temperature with a Perkin Elmer Lambda 25 UV-vis spectrometer against appropriate reagent blank. EPR spectra were obtained on a JEOL-JES FA200 ESR spectrometer in solid state at 298 K. Magnetic susceptibility measurements were made using a Magway MSB MKI Magnetic susceptibility balance (Sherwood Scientific Ltd, Cambridge, England) at 298 K. Data were corrected for diamagnetic contributions using Pascal's constants. TGA study was done by Perkin Elmer-Pyris Diamond TG/DTA instrument. NMR spectral measurements were carried out in CDCl3 solution at ambient temperature. The chemical shift was referenced to tetramethylsilane (TMS) from Bruker 300 MHz NMR spectrometer and the mass spectral analyses were done in methanol solvent from Waters Xevo® G2 QTof.Synthesis of [MnII(DEMP)(NCS)(H2O)]
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)], 1542 [ν(C–O)], 3418 [ν(O–H)]. UV-vis in MeOH [λmax nm (ε M−1 cm−1): 253 nm (6015), 275 (5147), 312 (2108), 405 (302)]. ESI-MS (+) in MeOH: m/z (relative intensity) 221.16 [M+, 100] (S3†). 1H NMR (300 MHz, CDCl3, δ ppm): 7.46–6.78 (4H, m, aromatic protons), 8.61 (1H, s, from Ph-OH). 13C NMR (75 MHz, CDCl3, δ ppm): 170.28, 161.33, 136.97, 133.45, 132.13, 117.62, 117.0, 77.43, 76.58, 50.67, 30.87.
N)], 1542 [ν(C–O)], 3418 [ν(O–H)]. UV-vis in MeOH [λmax nm (ε M−1 cm−1): 253 nm (6015), 275 (5147), 312 (2108), 405 (302)]. ESI-MS (+) in MeOH: m/z (relative intensity) 221.16 [M+, 100] (S3†). 1H NMR (300 MHz, CDCl3, δ ppm): 7.46–6.78 (4H, m, aromatic protons), 8.61 (1H, s, from Ph-OH). 13C NMR (75 MHz, CDCl3, δ ppm): 170.28, 161.33, 136.97, 133.45, 132.13, 117.62, 117.0, 77.43, 76.58, 50.67, 30.87.
The resulting solution on standing at room temperature produced brown micro-crystalline solid after 15 days. Yield: 76%. IR (KBr, cm−1): 1618.91[ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)], 2054.34 [ν(CNS)], 800.33 [ν(CS)], 3386.87 [ν(Mn–O)]. ESI-MS (+) in MeCN: m/z (relative intensity) 373.11 [M+ + Na]. UV-vis in DCM [λmax nm (ε M−1 cm−1): 298 (7583), 254 (14742), 632 (3529)] (S4†). 1H NMR (300 MHz, CDCl3, δ ppm): 7.40–6.33 (4H, m, aromatic protons). 13C NMR (75 MHz, CDCl3, δ ppm): 206.948, 131.34, 130.79, 119.6, 116.78, 116.30, 77.61, 77.46, 30.91. The compound is soluble in organic solvents like methanol, acetonitrile, chloroform, acetone, N,N-dimethyl formamide (DMF), dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF) and behaves like a non-electrolyte. Despite multiple attempts, diffractable grade suitable single crystals could not be obtained.
N)], 2054.34 [ν(CNS)], 800.33 [ν(CS)], 3386.87 [ν(Mn–O)]. ESI-MS (+) in MeCN: m/z (relative intensity) 373.11 [M+ + Na]. UV-vis in DCM [λmax nm (ε M−1 cm−1): 298 (7583), 254 (14742), 632 (3529)] (S4†). 1H NMR (300 MHz, CDCl3, δ ppm): 7.40–6.33 (4H, m, aromatic protons). 13C NMR (75 MHz, CDCl3, δ ppm): 206.948, 131.34, 130.79, 119.6, 116.78, 116.30, 77.61, 77.46, 30.91. The compound is soluble in organic solvents like methanol, acetonitrile, chloroform, acetone, N,N-dimethyl formamide (DMF), dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF) and behaves like a non-electrolyte. Despite multiple attempts, diffractable grade suitable single crystals could not be obtained.
Computational details
Ground state electronic structure calculations in methanol solution of both the ligand and complex have been carried out using DFT54 method associated with the conductor-like polarisable continuum model (CPCM).55–57 Becke's hybrid function58 with the Lee–Yang–Parr (LYP) correlation function59 was used through the study. The geometry of the ligand and complex was fully optimized without any symmetry constraints. On the basis of the optimized ground state geometry, the absorption spectral properties in methanol (CH3OH) media were calculated by time-dependent density functional theory (TDDFT)60–62 approach associated with the conductor-like polarisable continuum model (CPCM).55–57 We computed the lowest 20 singlet–singlet transition and results of the TD calculations were qualitatively very similar. The TDDFT approach had been demonstrated to be reliable for calculating spectral properties of many transition metal complexes.63–66 Due to the presence of electronic correlation in the TDDFT (B3LYP) method it can yield more accurate electronic excitation energies. Hence TDDFT had been shown to provide a reasonable spectral feature for our complex of investigation.We have run the Gaussian for geometry optimization of complex in ground state with three different basis sets namely 6-31G, 6-311G and 6-31++G(d,p) under B3LYP. For Mn atom, we used 6-31G + G(d,p), 6-311G+ G(d,p), 6-31+ +G(d,p), for H atoms we put 6-31G, 6-311G, 6-31G basis set; for C, N and O atoms we employed 6-31G, 6-311G, 6-31G and for S atom we adopt 6-31G + G(d,p), 6-311G + G(d,p), 6-31++G(d,p) as basis set under B3LYP and compared the results, which did not show any significant change in geometrical parameters. We have used 6-31G/B3LYP basis set for ligand optimization in ground state. All the calculations were performed with the Gaussian 09W software package.67 GaussSum 2.1 program68 was used to calculate the molecular orbital contributions from groups or atoms.
Fluorescence spectral study
The fluorescence spectra were measured on Elico SL 174 spectrofluorimeter following standard methods69,70 using the excitation wavelength of 280 nm and the emission was recorded from 280 nm to 900 nm. The fluorescence intensities of the ligand and the complex were monitored in different solvents like tetrahydrofuran (THF), N,N-dimethylformamide (DMF), dimethylsulfoxde (DMSO), dichloromethane (DCM), chloroform (CHCl3), acetone, acetonitrile and methanol as a function of concentration (10−4, 10−5, and 10−6 M) to observe the effects of the polarity on the photophysical activity of the complex and the ligand. The lifetimes were measured on Horiba Jobin Yvon single photon counter instrument.Conclusions
A new [MnII(DEMP)(NCS)(H2O)] complex has been designed and synthesized with the aim of developing a new light harvesting agent. The ability of the metal complex to absorb light over a wide region, from 200 nm to 850 nm, allows it to cover almost entirely the range of the sunlight falling on earth. The longer life times and strong fluorescence make the complex a suitable candidate for the development of new light harvesting device as well as a photoactive material.Acknowledgements
DG is thankful to UGC-BSR for providing JRF. Authors are thankful to UGC, New Delhi for financial support in the form of Major Research Project [Sanction no. F. no. 39-706/2010(SR)] to KKM. Financial support from DST FIST for the EPR equipment to the Department of Chemistry, Jadavpur University is also acknowledged.References
- C. Andreini, I. Bertini, G. Cavallaro, G. L. Holliday and J. M. Thornton, JBIC, J. Biol. Inorg. Chem., 2008, 13, 1205–1218 CrossRef CAS PubMed.
- J. Roth, S. Ponzoni and M. Aschner, Chapter 6 Manganese Homeostasis and Transport, in Metallomics and the Cell, ed. L. Banci, Metal Ions in Life Sciences 12, Springer, 2013 Search PubMed.
- W. F. Beyer, Jr and I. Fridovich, Biochemistry, 1985, 24, 6460–6467 CrossRef.
- S. Kamarajugadda, Q. Cai, H. Chen, S. Nayak, J. Zhu, M. He, Y. Jin, Y. Zhang, L. Ai, S. S. Martin, M. Tan and J. Lu, Cell Death Dis., 2013, 4, 504 CrossRef PubMed.
- S. Leitch, M. Feng, S. Muend, L. T. Braiterman, A. L. Hubbard and R. Rao, BioMetals, 2011, 24, 159–170 CrossRef CAS PubMed.
- Z. Liu, S. Li, Y. Cai, A. Wang, Q. He, C. Zheng, T. Zhao, X. Ding and X. Zhou, Free Radical Biol. Med., 2012, 53, 44–50 CrossRef CAS PubMed.
- R. M. Fronko, J. E. Penner-Hahn and C. J. Bender, J. Am. Chem. Soc., 1988, 110, 7554–7555 CrossRef CAS.
- A. R. Cyr, K. E. Brown, M. L. McCormick, M. C. Coleman, A. J. Case, G. S. Watts, B. W. Futscher, D. R. Spitz and F. E. Domann, Redox Biology, 2013, 1, 172–177 CrossRef CAS PubMed.
- A. Ramachandran, M. Lebofsky, S. A. Weinman and H. Jaeschke, Toxicol. Appl. Pharmacol., 2011, 251, 226–233 CrossRef CAS PubMed.
- R. Radmer and G. Cheniae, Primary Processes of Photosynthesis, in Topics Photosynth., ed. J. Barber, Elsevier, Amsterdam, New York, 1977, pp. 303–348 Search PubMed.
- S. Shcolnick and N. Keren, Plant Physiol., 2006, 141, 805–810 CrossRef CAS PubMed.
- B. A. Diner and P. Joliot, Encycl. Plant Physiol., New Ser., 1977, 5, 187–205 CAS.
- A. Pirson, Nutritional and metabolic studies on physiological Fontinalis and chlorella, Zeils. bot., 1937, 31, 193–267 CAS.
- E. Kessler, W. Arthur and J. E. Brugger, Arch. Biochem. Biophys., 1957, 71, 326–335 CrossRef CAS PubMed.
- Y. Umena, K. Kawakami, J. R. Shen and N. Kamiya, Nature, 2011, 473, 55–60 CrossRef CAS PubMed.
- A. Grundmeier and H. Dau, Biochim. Biophys. Acta, 2012, 1817, 88–105 CrossRef CAS PubMed.
- G. M. Cheniae and I. F. Martin, Biochim. Biophys. Acta, 1970, 197, 219–239 CrossRef CAS.
- M. M. Najafpour, J. Photochem. Photobiol., B, 2011, 104, 111–117 CrossRef CAS PubMed.
- J. S. Kanady, E. Y. Tsui, M. W. Day and T. Agapie, Science, 2011, 333, 733–736 CrossRef CAS PubMed.
- G. Christou, Acc. Chem. Res., 1989, 22, 328–335 CrossRef CAS.
- R. J. Debus, Biochim. Biophys. Acta, 1992, 1102, 269–390 CrossRef CAS.
- G. Renger, Topics in Photosynthesis, the Photosystems: Structure, Function and Molecular Biology, ed. J. Barber, Elsevier, Amsterdam, 1992, p. 45 Search PubMed.
- T. M. Bicker and D. F. Ghanotakis, Oxygenic Photosynthesis: The Light Reactions, ed. D. R. Ort and C. F. Yocum, Kluwer Academic, Dordrecht, 1996, p. 113 Search PubMed.
- A. Harriman, Coord. Chem. Rev., 1979, 28, 147–175 CrossRef CAS.
- K. Sauer and V. K. Yachandra, Biochim. Biophys. Acta, 2004, 1655, 140–148 CrossRef CAS PubMed.
- H. Isobe, M. Shoji, K. Koizumi, Y. Kitagawa, S. Yamanaka, S. Kuramitsu and K. Yamaguchi, Polyhedron, 2005, 24, 2767–2777 CrossRef CAS.
- K. Yamaguchi, S. Yamanaka, H. Isobe, M. Shoji, K. Koizumi, Y. Kitagawa, T. Kawakami and M. Okumura, Polyhedron, 2007, 26, 2216–2224 CrossRef CAS.
- P. Kurz, G. Berggren, M. F. Anderlund and S. Styring, Dalton Trans., 2007, 38, 4258–4261 RSC.
- K. Wieghardt, Angew. Chem., Int. Ed. Engl., 1989, 28, 1153–1172 CrossRef.
- M. M. Najafpour, T. Ehrenberg, M. Wiechen and P. Kurz, Angew. Chem., Int. Ed., 2010, 49, 2233–2237 CrossRef CAS PubMed.
- A. K. Poulsen, A. Rompel and C. J. McKenzie, Angew. Chem., Int. Ed., 2005, 44, 6916–6920 CrossRef CAS PubMed.
- V. L. Pecoraro, M. J. Baldwin and A. Gelasco, Chem. Rev., 1994, 94, 807–826 CrossRef CAS.
- L. Sun, L. Hammarström, B. Åkermark and S. Styring, Chem. Soc. Rev., 2001, 30, 36–49 RSC.
- A. Magnuson, M. Anderlund, O. Johansson, P. Lindblad, R. Lomoth, T. Polivka, S. Ott, K. Stensjö, S. Styring, V. Sundström and L. Hammarström, Acc. Chem. Res., 2009, 42, 1899–1909 CrossRef CAS PubMed.
- M. Yagi and K. Narita, J. Am. Chem. Soc., 2004, 126, 8084–8085 CrossRef CAS PubMed.
- M. Hakala, I. Tuominen, M. Keranen, T. Tyystjarvi and E. Tyystjarvi, Biochim. Biophys. Acta, 2005, 1706, 68–80 CrossRef CAS PubMed.
- R. Brimblecombe, A. Koo, G. C. Dismukes, G. F. Swiegers and L. Spiccia, J. Am. Chem. Soc., 2010, 132, 2892–2894 CrossRef CAS PubMed.
- R. Croce and H. van Amerongen, J. Photochem. Photobiol., B, 2011, 104, 142–153 CrossRef CAS PubMed.
- S. Belaid, A. Landreau, S. Djebbar, O. Benali-Baitich, G. Bouet and J.-P. Bouchara, J. Inorg. Biochem., 2008, 102, 63–69 CrossRef CAS PubMed.
- G. H. Jeffery, J. Bassett, J. Mendham and R. C. Denny Addison, Vogel's Text Book of Quantitative Chemical Analysis, Wesley Longman Limited, UK, 5th edn, 1989 Search PubMed.
- R. Biswas, M. G. B. Drew, C. Estarellas, A. Frontera and A. Ghosh, Eur. J. Inorg. Chem., 2011, 2011, 2558–2566 CrossRef.
- S. Naiya, M. G. B. Drew, C. Diaz, J. Ribas and A. Ghosh, Eur. J. Inorg. Chem., 2011, 2011, 4993–4999 CrossRef CAS.
- M. J. Frisch, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- J. Delaunay and R. P. Hugel, Inorg. Chem., 1986, 25, 3957–3961 CrossRef CAS.
- R. J. H. Clark and C. S. Williams, Spectrochim. Acta, 1966, 22, 1081–1090 CrossRef CAS.
- B. Mabad, P. Cassoux, J. P. Tuchagues and D. N. Hendrickson, Inorg. Chem., 1986, 25, 1420–1431 CrossRef CAS.
- C. Hureau, E. Anxolabèhère-Mallart, M. Nierlich, F. Gonnet, E. Rivière and G. Blondin, Eur. J. Inorg. Chem., 2002, 2710–2719 CrossRef CAS.
- L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59–83 CrossRef CAS.
- L. A. Cabell, M. D. Best, J. J. Lavigne, S. E. Schneider, D. M. Perreault, M.-K. Monahan and E. V. Anslyn, J. Chem. Soc., Perkin Trans. 2, 2001, 315–323 RSC.
- S. F. Forgues and D. Lavabre, J. Chem. Educ., 1999, 76, 1260–1264 CrossRef.
- M. Sarkar, S. S. Paul and K. K. Mukherjea, J. Lumin., 2013, 142, 220–230 CrossRef CAS.
- A. K. Satpati, S. Senthilkumar, M. Kumbhakar, S. Nath, D. K. Maity and H. Pal, Photochem. Photobiol., 2005, 81, 270–278 CrossRef CAS PubMed.
- S. Mukherjee, J. A. Stullb, J. Yanoc, T. C. Stamatatosa, K. Pringouria, T. A. Stichb, K. A. Abbouda, R. David Brittb, V. K. Yachandrac and G. Christoua, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2257–2262 CrossRef CAS PubMed.
- R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, Oxford, 1989 Search PubMed.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- M. Cossi and V. Barone, J. Chem. Phys., 2001, 115, 4708–4717 CrossRef CAS.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- M. E. Casida, C. Jamorski, K. C. Casida and D. R. Salahub, J. Chem. Phys., 1998, 108, 4439–4449 CrossRef CAS.
- R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218–8224 CrossRef CAS.
- R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS.
- T. Liu, H.-X. Zhang and B.-H. Xia, J. Phys. Chem. A, 2007, 111, 8724–8730 CrossRef CAS PubMed.
- X. P. Zhou, H.-X. Zhang, Q.-J. Pan, B.-H. Xia and A.-C. Tang, J. Phys. Chem. A, 2005, 109, 8809–8818 CrossRef CAS PubMed.
- X. P. Zhou, A.-M. Ren and J.-K. Feng, J. Organomet. Chem., 2005, 690, 338–347 CrossRef CAS.
- A. Albertino, C. Garino, S. Ghiani, R. Gobetto, C. Nervi, L. Salassa, E. Rosenverg, A. Sharmin, G. Viscardi, R. Buscaino, G. Cross and M. Milanesio, J. Organomet. Chem., 2007, 692, 1377–1391 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, (Revision A.1), Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- N. M. O'Boyle, A. L. Tenderholt and K. M. Langner, J. Comput. Chem., 2008, 29, 839–845 CrossRef PubMed.
- S. S. Paul, M. Selim, A. Saha and K. K. Mukherjea, Dalton Trans., 2014, 43, 2835–2848 RSC.
- S. Patra, S. Chatterjee, T. K. Si and K. K. Mukherjea, Dalton Trans., 2013, 42, 13425–13435 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00729h |
| This journal is © The Royal Society of Chemistry 2014 |