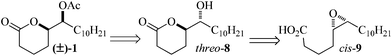
Diastereodivergent total synthesis of mosquito oviposition pheromone†
David Hurem and
Travis Dudding*
Department of Chemistry, Brock University, 500 Glenridge Avenue, St. Catharines, L2S 3A1, ON, Canada. E-mail: tdudding@brocku.ca
First published on 17th March 2014
Abstract
The unnatural threo-6-acetoxy-5-hexadecanolide and the natural mosquito oviposition pheromone erythro-6-acetoxy-5-hexadecanolide were synthesized in a diastereodivergent fashion in 44% and 33% overall yield respectively from 5-bromovaleric acid and undecanal. The key step utilized a chemoenzymatic epoxidation-lactonization of a naturally available fatty acid to form the 6-hydroxy-5-hexadecanolide core.
Introduction
A series of experiments initiated in 1982 by Pickett and coworkers led to the identification of (5R,6S)-6-acetoxy-5-hexadecanolide (1) as the major chemical component of Culex sp. egg rafts responsible for the potent and selective attraction of gravid Culex mosquitos (Fig. 1).1Mosquito species of the Culex genus comprise the majority of vectors responsible for the transmission of encephalitic arboviruses, such as West Nile virus.2 In order to monitor these diseases, Mosquito Oviposition Pheromone (MOP) 1 has become a sought after attractant for the surveillance of arbovirus occurrence within mosquito populations, due to its selectivity for gravid mosquitos that have had a chance to become laterally infected.3 Apart from its use in mosquito surveillance, 1 has also been used in conjunction with larvacides as an alternative method of pest control.4 Accordingly, the existing demand for this natural product has generated over twenty total syntheses of 1 and its corresponding stereoisomers since Mori's total synthesis in 1983.5–8,9a,c Although various enantioselective syntheses have afforded sufficient quantities of 1 and its corresponding stereoisomers to allow for the elucidation of their specific biological activities, these methods are of limited practicality for the large scale production and use of optically enriched 1 in control or surveillance applications. Notwithstanding, biological activity assays indicated that the non-natural stereoisomers (ent-1, 2 and ent-2, Fig. 1) were inactive, but not repulsive,1c,e allowing for the use of racemic mixtures of 1 and ent-1 as attractants in oviposition traps.9 In view of this fact, it is important to note that bioactive plant oil containing a racemic mixture of 1 and ent-1 can be produced from Kochia scoparia seed extract that initially contained ∼25% (Z)-5-hexadecenoic acid, thus offering a renewable source for the production of oviposition attractant.9a,b
The most successful strategies for the synthesis of racemate (±)-1 have involved either aldol addition of cyclopentanone to undecylaldehyde followed by Bayer–Villiger oxidation and acetylation (route A, Scheme 1),6 or dihydroxylation of (Z)-5-hexadecenoic acid (7) or the methyl ester of 7 followed by intramolecular cyclization to form the erythro-hydroxyalkanolide precursor to (±)-1 (route B, Scheme 1).9a,c
Both strategies lead to a concise synthesis of racemic erythro-6-acetoxy-5-hexadecanolide (±)-1 with high overall yields (∼65%). Utilizing route B, product (±)-1 was obtained from the naturally available acid 7 via the dihydroxylation route; unfortunately this transformation required the use of stoichiometric heavy metal reagents. Alternatively, route A was recently employed to access enantioenriched 1 and 2 using the Hajos–Parish asymmetric aldol reaction,7,8 however the large excess of m-CPBA (∼5 eq.) for Bayer–Villiger oxidation detracted from this procedure. Apart from the hazardous reagents required for these oxidations, the necessity for purification by column chromatography at each step of the synthesis greatly limited the scalability of these reactions.
To overcome the limitations associated with the above oxidations and at the same time incorporate salient features of routes A and B in Scheme 1, it was reasoned that a more ideal synthesis would intersect naturally available acid 7 and proceed through a benign, metal-free oxidation. Epoxidations of olefins, mediated by the in situ lipase catalyzed generation of peroxyacids using aqueous hydrogen peroxide as a primary oxidant have been reported in the literature and would offer one means to this end.10 It was also understood that this very mild oxidation procedure would yield the hydroxylactone threo-8, after which the relative geometry at C(6) could be inverted by esterification to afford (±)-1 (Scheme 2). Accordingly, the total synthesis of (±)-1 via an environmentally benign lipase mediated epoxidation of (Z)-5-hexadecenoic acid 7 is reported. Marked features of this synthesis include the minimization of column purification by using urea inclusion crystallization and favourable ‘Green’ metrics.
Results and discussion
At the outset, fatty acid 7 was synthesized by Wittig olefination under kinetic conditions to favour the (Z)-isomer (Scheme 3), as determined by characteristic signals from 1H and 13C NMR.11 Although Wittig olefination cannot be considered green, the reaction was chosen as a facile route to pure 5-hexadecenoic acid 7, as a proof of principle for the synthesis of (±)-1 from the naturally available fatty acid. | ||
| Scheme 3 Synthesis of fatty acid precursor using a Conia–Dauben modified Wittig reaction under kinetic conditions. | ||
While investigating choice of base, it was found that the use of sodium t-amyloxide, in a similar manner to that described by Dauben et al.,12 resulted in higher yields and cleaner workup than analogous reactions with potassium t-butoxide in THF (see ESI† for detailed experimental procedure) (Table 1). Purification methods for the removal of triphenylphosphine oxide (TPPO) were then investigated. The majority of TPPO remained in the organic reaction medium, however ∼25% of TPPO was carried over in the acid/base extraction resulting in a significant TPPO impurity in the crude oil extract. The use of column purification proved challenging, requiring a gradient mobile phase starting with hexanes, then hexanes–ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce colourless fatty acid in 61% yield at a maximum 100 mg scale. To overcome difficulties with large scale column purification, the gram scale (1–5 g) purification of extract was achieved using urea inclusion crystallization.13 This technique allowed for the isolation of up to 3 g of clear colourless fatty acid material that was free of TPPO and analytically pure in 48% overall yield and retention of the (Z)-stereochemistry, while producing only aqueous urea solution and a small volume of TPPO in methanol as waste.
1) to produce colourless fatty acid in 61% yield at a maximum 100 mg scale. To overcome difficulties with large scale column purification, the gram scale (1–5 g) purification of extract was achieved using urea inclusion crystallization.13 This technique allowed for the isolation of up to 3 g of clear colourless fatty acid material that was free of TPPO and analytically pure in 48% overall yield and retention of the (Z)-stereochemistry, while producing only aqueous urea solution and a small volume of TPPO in methanol as waste.
| Entry | Base | Solvent | Urea yielda (%) | Column yieldb (%) | Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ec Ec |
|---|---|---|---|---|---|
| a Yield of reactions purified using two serial urea inclusion crystallizations at 5 g scale.b Yields of reactions purified by column chromatography at 100 mg scale.c Ratios were estimated using 1H NMR.11 | |||||
| 1 | t-BuOK | THF | 32 | 45 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | t-AmONa | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (1 toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
74 | — | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
With the requisite fatty acid in hand, the lipase mediated Prilezhaev oxidation of the olefin was investigated (Scheme 4). Initially the incremental addition of hydrogen peroxide to a 100 mM solution of fatty acid 7 in cyclohexane over immobilized lipase resulted in a poor combined yield (30%) of hydroxylactone and epoxyacid (Table 2, entry 1). Previously, it was reported that the epoxyacid 9 undergoes oxidation to the peracid at a much slower rate than the unsaturated acid 7, while the formation of peracid is much faster than the epoxidation.10a Accordingly, it was reasoned that an incremental addition of the fatty acid would maintain higher concentrations of the unsaturated fatty acid 7 during the progress of the reaction, which ultimately resulted in complete consumption of fatty acid as observed by TLC after 48 hours with minimal formation of by-products (Table 2, entry 2).
| Entry | Addition time of H2O2a (h) | Aliquots of fatty acid 8b | No. of lipase reusesc | Yieldd (%) |
|---|---|---|---|---|
| a Aqueous H2O2 (15 M) was added over 24 h via syringe pump.b Aliquots of a solution of 8 (0.21 M) in cyclohexane were added to the reaction in 6 h increments.c Fatty acid 8 (1.5 mmol) was epoxidized over 48 h using the same 126 mg of lipase (CALB Immobead 150) for each reuse.d Represents yield of hydroxylactone 9. | ||||
| 1 | 24 | 1 | 0 | 30 |
| 2 | 24 | 4 | 0 | 69 |
| 3 | 24 | 4 | 1 | 68 |
| 4 | 24 | 4 | 2 | 69 |
Under the room temperature oxidation conditions as described above, the epoxyacid 9 was formed along with ∼10% hydroxylactone 8 as observed by crude 1H NMR. Complete lactonization was then achieved by heating the cyclohexane solution of 8 and 9 under reflux. Further optimization revealed that the addition of 0.04% (v/v) Et3N significantly reduced the time required for lactonization from 72 h to 12 h, likely due to the base acting as a proton shuttle. The diastereomerically pure threo-hydroxylactone 8 was isolated in 40% yield by crystallization from hexanes, and the remaining hydroxylactone 8 was obtained from flash chromatography of the mother liquor and subsequent crystallization for a combined yield of 65%. With these working experimental conditions, the ability to reuse the immobilized lipase was investigated, which revealed that the procedure could be repeated up to three times with no apparent loss of activity, so long as reaction runs were repeated immediately after filtration (Table 2, entry 2–4). Whereas storage of the used immobilized lipase for one week at 7 °C unfortunately resulted in complete loss of activity.
The synthesis was completed by way of a late diastereodivergent acetylation strategy. To this end, the threo-12 was synthesized by O-acetylation of 8 using standard conditions (Scheme 5a).8 Conversely, the (±)-1 was synthesized by mesylation of the C(6) hydroxyl, followed by substitution of the resulting mesylate with acetate (Scheme 5b).5h
At this stage the reaction metrics of the key oxidation steps and the overall synthesis, including several commonly used green metrics were determined for this synthesis and compared with two of the more successful racemic syntheses.14 Due to the fact that current ‘Green’ metrics fail to account for every possible parameter that influences the environmental impact of a process, no one metric can be applied to assess the sustainability of a given process. As such, a series of green metrics were selected, along with standard reaction metrics like yield and steps, to compare the presented racemic synthesis with successful syntheses from the literature. Two metrics relating to mass efficiency were selected; (1) Sheldon's E-factor was selected as an easy measure of relative waste and (2) Glaxo-Smith-Klein's (GSK) metric was used to gauge reaction mass efficiency. Carbon efficiency (CE) was also calculated to gauge the efficiency of the transfer of organic material in this synthesis. E-factors and RME were calculated for each step of the synthesis by eqn (1) and (2) respectively, such that msm is the mass of starting materials and mp is the mass products.14
 | (1) |
 | (2) |
The mass of all consumable starting reagents and catalysts were incorporated, while solvents were considered recoverable and aqueous solutions were considered benign, and therefore excluded from the calculation. These assumptions were made for the sake of comparing syntheses at the bench scale, where solvent choices, quantities and lifecycles can be expected to change significantly during scale up to an industrial process. Silica and urea were considered recoverable and were not factored into the equation as well. CE was determined according to eqn (3), such that np is the moles of product, nsm is the moles of each reagent, Csm is the number of carbons of that reagent and Cp is the number of carbons in the product.
 | (3) |
The reaction and green metrics of this reaction were then compared with those of the previous successful conventional syntheses.6,9c The reaction metrics for the key oxidation step reveal that the lipase mediated reaction is far more efficient in terms of mass efficiency despite its lower yield compared to oxidation with osmium tetroxide (Table 3). Although the dihydroxylation proceeded with high carbon efficiency, the use of excess inorganic salts lowered its RME and increased its E-factor significantly. Meanwhile, Bayer–Villiger oxidation was more ‘Green’ than that of dihydroxylation, while the lipase epoxidation was more mass and carbon efficient. Notwithstanding, apart from its moderate yield, the lipase oxidation appeared quite desirable in comparison to the other known techniques, especially since toxic osmium reagents and m-CPBA were replaced with aqueous hydrogen peroxide. Although cyclohexane was used as a solvent, it was successfully recovered by distillation (∼95% recovery), and reused in subsequent reaction runs.
Finally, the E-factor for the overall process was determined as a sum of E-factors at each step, while the overall RME and CE was calculated as the product of each RME and CE in the linear sequence. The overall synthesis scored lower in comparison to the oxidation alone (Table 4). Although the overall synthesis was more mass and carbon efficient than that proposed by Michaelakis et al., yields were half that of either synthesis. The synthesis of Dawson et al. was the most mass and carbon efficient between the three. However, the nature of reagents and reaction conditions cannot be accounted for by the available green metrics. As such, metrics can only serve to aid in a qualitative assessment of the relative sustainability of different processes or reactions when placed into the context of what is known about the environmental impact of the different reagents used in the respective processes or reactions.
Experimental
Materials and methods
Reagents and solvents were purchased from Sigma-Aldrich at the highest available reagent grade purity, and used without further purification unless otherwise stated. Oven-dried glassware was used in all experiments unless otherwise stated.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 toluene
1 toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (38 ml), at 0 °C, under a nitrogen atmosphere and with moderate to fast stirring with magnetic stir bar. To the resulting viscous orange suspension was added undecanal (2.54 g, 14.9 mmol) over 10 minutes with vigorous stirring under the conditions described above. The reaction was allowed to warm to room temperature overnight. The cream coloured mixture was extracted with H2O (4 × 10 ml). The combined aqueous phase was cooled in an ice bath and slowly brought to pH ∼2 by dropwise addition of aqueous 2% H2SO4 solution, extracted with ether (3 × 10 ml), combined organic layers were dried (MgSO4) and concentrated to a clear yellow oil (3.62 g). The crude oil was dissolved in methanol (6.6 ml) and transferred hot to a boiling solution of urea in methanol (16.5 g in 26 ml). The solution was allowed to crystallize overnight then filtered. The collected urea crystals were dissolved in 2% H2SO4, extracted with ether (3 × 10 ml), dried (MgSO4), and ether was removed in vacuo to yeild 7 as clear, nearly colourless oil containing about 10% aromatic impurities by 1H NMR (2.78 g). The analytically pure fatty acid was obtained by repeating the above process to yield 7 as a clear, colourless oil (2.56 g, 74%, Z
THF (38 ml), at 0 °C, under a nitrogen atmosphere and with moderate to fast stirring with magnetic stir bar. To the resulting viscous orange suspension was added undecanal (2.54 g, 14.9 mmol) over 10 minutes with vigorous stirring under the conditions described above. The reaction was allowed to warm to room temperature overnight. The cream coloured mixture was extracted with H2O (4 × 10 ml). The combined aqueous phase was cooled in an ice bath and slowly brought to pH ∼2 by dropwise addition of aqueous 2% H2SO4 solution, extracted with ether (3 × 10 ml), combined organic layers were dried (MgSO4) and concentrated to a clear yellow oil (3.62 g). The crude oil was dissolved in methanol (6.6 ml) and transferred hot to a boiling solution of urea in methanol (16.5 g in 26 ml). The solution was allowed to crystallize overnight then filtered. The collected urea crystals were dissolved in 2% H2SO4, extracted with ether (3 × 10 ml), dried (MgSO4), and ether was removed in vacuo to yeild 7 as clear, nearly colourless oil containing about 10% aromatic impurities by 1H NMR (2.78 g). The analytically pure fatty acid was obtained by repeating the above process to yield 7 as a clear, colourless oil (2.56 g, 74%, Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E = 8
E = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, E = 3.6). 1H NMR (600 MHz, CDCl3): δ 11.5 (s, 1H), 5.46–5.32 (m, 2H), 2.36 (t, J = 6.7 Hz, 2H), 2.14–2.01 (m, 4H), 1.74–1.70 (m, 2H), 1.35–1.10 (m, 18H), 0.92–0.87 (t, J = 6.7 Hz, 3H); 13C NMR (600 MHz, CDCl3): δ 180.5, 131.3, 128.1, 33.4, 31.9, 29.8–29.2, 27.3, 26.5, 24.5, 22.6, 14.1; IR (KBr pellet): ν 3500–2500, 2920, 2856, 1710, 1460, 1410, 935 cm−1; HRMS (FAB): m/z calcd for C16H30O2 [M + H]+, 255.2324; found, 255.2304.
2, E = 3.6). 1H NMR (600 MHz, CDCl3): δ 11.5 (s, 1H), 5.46–5.32 (m, 2H), 2.36 (t, J = 6.7 Hz, 2H), 2.14–2.01 (m, 4H), 1.74–1.70 (m, 2H), 1.35–1.10 (m, 18H), 0.92–0.87 (t, J = 6.7 Hz, 3H); 13C NMR (600 MHz, CDCl3): δ 180.5, 131.3, 128.1, 33.4, 31.9, 29.8–29.2, 27.3, 26.5, 24.5, 22.6, 14.1; IR (KBr pellet): ν 3500–2500, 2920, 2856, 1710, 1460, 1410, 935 cm−1; HRMS (FAB): m/z calcd for C16H30O2 [M + H]+, 255.2324; found, 255.2304.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane–EtOAc 0.02% Et3N) to yield 8 as a white solid (79 mg, 21%). The two were combined to yield 8 as a white powder (275 mg, 69%, E = 0.4) m.p.: 65–67 °C; 1H NMR (300 MHz, CDCl3): δ 4.2 (m, 1H), 3.6 (m, 1H), 2.6–2.5 (m, 2H), 2.0–1.2 (m, 22H), 0.9 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 171.6, 83.2, 73.3, 32.6, 31.9, 29.5, 25.4, 24.2, 22.7, 18.4, 14.1; IR (KBr pellet): ν 3554 (br), 2955, 1706 cm−1; HRMS (FAB): m/z calcd for C16H30O3 [M + H]+ 271.2273, found 271.2258.
1 hexane–EtOAc 0.02% Et3N) to yield 8 as a white solid (79 mg, 21%). The two were combined to yield 8 as a white powder (275 mg, 69%, E = 0.4) m.p.: 65–67 °C; 1H NMR (300 MHz, CDCl3): δ 4.2 (m, 1H), 3.6 (m, 1H), 2.6–2.5 (m, 2H), 2.0–1.2 (m, 22H), 0.9 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 171.6, 83.2, 73.3, 32.6, 31.9, 29.5, 25.4, 24.2, 22.7, 18.4, 14.1; IR (KBr pellet): ν 3554 (br), 2955, 1706 cm−1; HRMS (FAB): m/z calcd for C16H30O3 [M + H]+ 271.2273, found 271.2258.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane–EtOAc 0.01% Et3N) to yield (±)-2 as a clear colourless oil (392 mg, 98%, E = 0.3). 1H NMR (300 MHz, CDCl3): δ 5.00 (m, 1H), 4.37 (dt, J = 4.5, 3.6, 1H), 2.60–2.47 (m, 2H), 2.11 (s, 3H), 2.01–1.50 (m, 6H), 1.27 (s, 16H) 0.9 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 170.9, 170.7, 79.8, 73.9, 31.9, 29.9–29.3, 25.3, 24.1, 22.7, 21.0, 18.4, 14.1; IR (KBr): cm−1; HRMS (EI): m/z calcd for C18H32O4 [M ]+ 312.2301, found 312.2313.
1 hexane–EtOAc 0.01% Et3N) to yield (±)-2 as a clear colourless oil (392 mg, 98%, E = 0.3). 1H NMR (300 MHz, CDCl3): δ 5.00 (m, 1H), 4.37 (dt, J = 4.5, 3.6, 1H), 2.60–2.47 (m, 2H), 2.11 (s, 3H), 2.01–1.50 (m, 6H), 1.27 (s, 16H) 0.9 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 170.9, 170.7, 79.8, 73.9, 31.9, 29.9–29.3, 25.3, 24.1, 22.7, 21.0, 18.4, 14.1; IR (KBr): cm−1; HRMS (EI): m/z calcd for C18H32O4 [M ]+ 312.2301, found 312.2313.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane–EtOAc 0.02% Et3N) to afford (±)-1 as a clear colourless oil (139.8 mg, 73%, E = 4.5). 1H NMR (300 MHz, CDCl3): δ 4.95 (m, 1H), 4.32 (m, 1H), 2.56–2.39 (m, 2H), 2.04 (s, 3H), 2.01–1.70 (m, 3H), 1.62 (m, 3H) 1.22 (s, 16H) 0.84 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 170.8, 170.4, 80.5, 74.2, 31.8, 29.6–29.3, 25.2, 23.4, 22.6, 21.0, 18.3, 14.1; HRMS (EI): m/z calcd for C18H32O4 [M]+ 312.2301, found 312.2303.
1 hexane–EtOAc 0.02% Et3N) to afford (±)-1 as a clear colourless oil (139.8 mg, 73%, E = 4.5). 1H NMR (300 MHz, CDCl3): δ 4.95 (m, 1H), 4.32 (m, 1H), 2.56–2.39 (m, 2H), 2.04 (s, 3H), 2.01–1.70 (m, 3H), 1.62 (m, 3H) 1.22 (s, 16H) 0.84 (t, J = 6.7 Hz, 3H); 13C NMR (300 MHz, CDCl3): δ 170.8, 170.4, 80.5, 74.2, 31.8, 29.6–29.3, 25.2, 23.4, 22.6, 21.0, 18.3, 14.1; HRMS (EI): m/z calcd for C18H32O4 [M]+ 312.2301, found 312.2303.Conclusions
The diastereoselective synthesis of the biologically active erythro-6-acetoxy-5-hexadecanolide was achieved in 33% overall yield using a benign chemoenzymatic domino epoxidation-lactonization procedure. The E-factor for this oxidation/cyclization process was ∼0.4, while the E-factor for the overall synthesis starting from 5-bromovaleric acid and undecanal was ∼9, whereas E-factors in pharmaceutical production range between 25 and 100.14a,b A technique used in industrial purification of plant oils was demonstrated as a practical means of purifying a fatty acid from Wittig reactions on a gram scale. The threo-diastereomer was synthesized in 44% overall yield.At this stage the reliance on conventional means for the synthesis of fatty acid 7, and acetylation to afford (±)-1 has, as illustrated by E-factors provided in the experimental, introduced the largest volume of waste, and required the use of non-benign solvents. The benign oxidation procedure utilized in this work has never-the-less significantly improved upon the ‘greeness’ of the synthesis from previous attempts that also relied on such non-benign solvents as DCM and toluene.
Ongoing work is focussed on applying asymmetric methodologies to add to the overall utility of this approach. Moreover, in keeping within the tenets of green chemistry,15 catalytic methods for the stereoinversion of the C(6) center are being investigated. At the same time, starting fatty acid sources from biological avenues are being explored. The overall long term aims of this research effort are to further improve the synthesis of 1 with reduced overall waste output to render its use in mosquito control applications more feasible.
Acknowledgements
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding this research.Notes and references
- (a) D. W. Bruno and B. R. Laurence, J. Med. Entomol., 1979, 16, 300 CrossRef; (b) B. R. Laurence and J. A. Pickett, J. Chem. Soc., Chem. Commun., 1982, 59 RSC; (c) K. Mori and T. Otsuka, Tetrahedron, 1983, 39, 3267 CrossRef CAS; (d) B. R. Laurence, K. Mori, T. Otsuka, J. A. Pickett and L. J. Wadhams, J. Chem. Ecol., 1985, 11, 643 CrossRef CAS PubMed; (e) Y.-S. Hwang, M. S. Mulla, J. D. Chaney, G.-G. Lin and H.-J. Xu, J. Chem. Ecol., 1987, 13, 245 CrossRef CAS PubMed.
- R. S. Nasci and B. R. Miller, in The biology of disease vectors, ed. B. J. Beaty and W. C. Marquardt, University Press of Colorado, Colorado, 1996, pp. 85–97 Search PubMed.
- (a) W. K. Reisen and A. R. Pfuntner, J. Am. Mosq. Control Assoc., 1987, 3, 601 CAS; (b) S. A. Allan and D. J. Kline, J. Vector Ecol., 2004, 29, 285 Search PubMed; (c) J. L. Hardy, E. J. Houk, L. D. Kramer and W. C. Reeves, Annu. Rev. Entomol., 1983, 28, 229 CrossRef CAS PubMed.
- (a) S. M. Cook, Z. R. Khan and J. A. Pickett, Annu. Rev. Entomol., 2007, 52, 375 CrossRef CAS PubMed; (b) W. A. Otieno, T. O. Onyango, M. M. Pile, B. R. Laurence, G. W. Dawson, L. J. Wadhams and J. A. Pickett, Bull. Entomol. Res., 1988, 78, 463 CrossRef CAS.
- (a) K. J. Quinn, J. M. Curto, K. P. McGrath and N. A. Biddick, Tetrahedron Lett., 2009, 50, 7121 CrossRef CAS; (b) K. R. Prasad and P. Anbarasan, Tetrahedron: Asymmetry, 2007, 18, 2479 CrossRef CAS; (c) B. Dhotare, D. Goswami and A. Chattopadhyay, Tetrahedron Lett., 2005, 46, 6219 CrossRef CAS; (d) E. A. Couladouros and A. P. Mihou, Tetrahedron Lett., 1999, 40, 4861 CrossRef CAS; (e) B. B. Lohray and S. Venketaswarlu, Tetrahedron: Asymmetry, 1997, 8, 633 CrossRef CAS; (f) C. Bonini, M. Checconi, G. Righi and L. Rossi, Tetrahedron, 1995, 51, 4111 CrossRef CAS; (g) C. G. Pelletier, M. Sanier, I. Carvet, Y. Le Merrer and J. C. Depazay, Tetrahedron Lett., 1994, 35, 115 CrossRef; (h) H. Kotsuki, I. Kadota and O. Masamitsu, J. Org. Chem., 1990, 55, 4417 CrossRef CAS; (i) T. Kametani, M. Tsubuki, Y. Tatzuzaki and T. Honda, J. Chem. Soc., Perkin Trans. 1, 1990, 1, 639 RSC; (j) Z. M. Wang, X. H. Qian and W. S. Zhou, Tetrahedron, 1990, 46, 1191 CrossRef CAS; (k) S. K. Kang and I. H. Cho, Tetrahedron Lett., 1989, 30, 743 CrossRef CAS; (l) N. C. Barua and R. R. Schmidt, Tetrahedron, 1986, 42, 4471 CrossRef CAS; (m) K. Y. Ko and E. L. Eliel, J. Org. Chem., 1986, 51, 5353 CrossRef CAS; (n) G. G. Lin, H. J. Xu, B. C. Wu, G. Z. Guo and W. S. Zhou, Tetrahedron Lett., 1985, 26, 1233 CrossRef.
- W. G. Dawson, A. Mudd, J. A. Pickett, M. M. Pile and L. J. Wadhams, J. Chem. Ecol., 1990, 16, 1779 CrossRef PubMed.
- B. Sun, L. Peng, X. Chen, Y. Li, Y. Li and K. Yamasaki, Tetrahedron: Asymmetry, 2005, 16, 1305 CrossRef CAS.
- I. Hideaki, S. Yusuke, I. Yoshiyasu and H. Kotsuki, Tetrahedron, 2006, 62, 311 CrossRef.
- (a) T. O. Olagbemiro, M. A. Birkett, A. J. Mordue (Luntz) and J. A. Pickett, J. Agric. Food Chem., 1999, 47, 3411 CrossRef CAS PubMed; (b) T. O. Olagbemiro, M. A. Birkett, A. J. Mordue (Luntz) and J. A. Pickett, J. Chem. Ecol., 2004, 30, 965 CrossRef CAS PubMed; (c) A. Michaelakis, A. P. Mihou, E. A. Couladouros, A. K. Zounos and G. Koliopoulos, J. Agric. Food Chem., 2005, 53, 5225 CrossRef CAS PubMed.
- (a) S. Warwel and M. R. Klaas, J. Mol. Catal. B: Enzym., 1995, 29 CrossRef CAS; (b) F. Bjorkling, H. Frykman, S. E. Godtfredsen and O. Kirk, Tetrahedron, 1992, 48, 4587 CrossRef CAS.
- Carboxyl carbons of Z- and E-acids were resolved at 600 MHz on 13C NMR and integration ratio corresponded to the ratio of integrals of olefin multiplets at 600 MHz on 1H NMR, while 13C NMR signals corresponding to vinyl carbons were characteristic for (Z)-fatty acids. For common chemical shifts of monounsaturated fatty acids see: D. J. Frost and F. D. Gunstone, Chem. Phys. Lipids, 1975, 15, 53 CrossRef CAS PubMed.
- This methodology was originally reported in: W. G. Dauben and D. M. Walker, J. Org. Chem., 1981, 46, 1103 CrossRef CAS A detailed procedure was outlined in: R. P. Short, J. M. Revol, B. C. Ranu and T. Hudlicky, J. Org. Chem., 1983, 48, 4453 CrossRef.
- D. Swern and W. E. Parker, J. Am. Oil Chem. Soc., 1952, 29, 614 CrossRef CAS.
- (a) R. A. Sheldon, Chem. Commun., 2008, 3352 RSC; (b) R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC; (c) For a recent comparison of ‘Green’ metrics see: D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521 RSC; (d) For other ‘Green’ metrics see: T. Hudlicky, D. A. Frey, L. Koroniak, C. D. Claeboe and L. E. Brammer, Green Chem., 1999, 57 RSC; (e) B. M. Trost, Science, 1991, 254, 1471 CAS; (f) A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 30 Search PubMed.
Footnote |
† Electronic supplementary information (ESI) available: Supporting experimental procedures, determination of Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E ratios, NMR spectra of isolated products and synthetic intermediates. See DOI: 10.1039/c4ra01542h E ratios, NMR spectra of isolated products and synthetic intermediates. See DOI: 10.1039/c4ra01542h |
| This journal is © The Royal Society of Chemistry 2014 |