Synthesis of multifunctional lipid–polymer conjugates: application to the elaboration of bright far-red fluorescent lipid probes†
Abstract
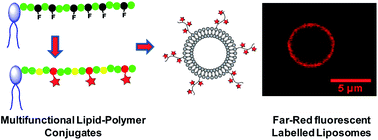
A new class of lipid-ended polymer conjugates presenting reactive sites regularly distributed along the polymer chain were synthesized using RAFT polymerization. The chosen modular approach enables preparation of different lipid families by tuning the nature of the phospholipid α-end, the molecular weight and the lateral functions of the polymer chain. The multiple activated ester functions of the conjugates can indeed be used for the efficient coupling of a great variety of amino-containing entities of interest. In this study, we elaborated original fluorescent lipid–polymer probes for optical microscopy by coupling along the chain a controlled number of chromophores emitting in the far-red where auto-fluorescence and light absorption by biological samples are limited. Water-soluble fluorescent lipid probes exhibiting an enhanced brightness were obtained. As a proof of concept, these probes were able to efficiently label the lipid bilayer of liposomes of various sizes. Such multifunctional lipid-ended polymers thus exhibit great potential to functionalize model and natural lipid assemblies.

 Please wait while we load your content...
Please wait while we load your content...