Dual-sulfonated dipyridinium phosphotungstate catalyst for liquid-phase Beckmann rearrangement of cyclohexanone oxime
Dan Mao,
Zhouyang Long,
Yu Zhou,
Jing Li,
Xiaochen Wang and
Jun Wang*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing 210009, Jiangsu, P. R. China. E-mail: junwang@njtech.edu.cn; Tel: +(86) 25-83172264
First published on 17th March 2014
Abstract
The heteropolyanion (HPA)-based ionic liquid (IL) hybrid was prepared by pairing Keggin-structured HPA of the phosphotungstate anion PW12O403− (PW) with dual-sulfonated 4,4′-dipyridinium IL-cation [DPySO3H]2+. The obtained powdered IL–HPA hybrid material [DPySO3H]1.5PW was characterized and catalytically assessed in Beckmann rearrangement of cyclohexanone-oxime to ε-caprolactam. Under the optimized reaction conditions: 10 mol% catalyst, 130 °C, 2 h, benzonitrile as the solvent, and without the environmentally harmful cocatalyst ZnCl2, [DPySO3H]1.5PW exhibits 100% conversion and 73.0% selectivity, remarkably higher than control catalysts involving other HPA-anions or the partially proton-substituted IL-cation. Moreover, the catalyst [DPySO3H]1.5PW can be isolated and reused via filtration from the liquid–solid heterogeneous reaction mixture demonstrated by the four-run recycling test.
1. Introduction
ε-Caprolactam is the starting material for manufacturing nylon fibers and resins which are widely used in textile, electronic and automobile industries. Beckmann rearrangement of cyclohexanone oxime over homogeneous oleum or sulfuric acid catalyst is currently the commercial process to produce ε-caprolactam, which inevitably has severe problems in equipment corrosion, product separation and a large amount of by-product ammonium sulphate.1,2 To resolve these problems, various catalysts have been investigated for this reaction in either vapour or liquid processes. The vapour-phase process over various solid acid catalysts is highly energy-consuming because it requires high temperatures above 300 °C.3–7 Therefore, low-temperature liquid-phase rearrangement has attracted much attention. In this area, organocatalysts, such as 1-chloro-2,3-diphenylcyclopropenium ion,8 cyanuric chloride,9 bis(2-oxo-3-oxazolidinyl)-phosphinic chloride (BOP-Cl)10 and so forth, exhibit good yields to ε-caprolactam at relatively low temperatures, but most of them involve environmental harmful halogen plus difficulty in catalyst isolation. It is thus very interesting to explore easily recoverable solid catalysts for the low-temperature liquid-phase Beckmann rearrangement of cyclohexanone oxime. Indeed, H-Beta zeolite,11 tungstated zirconia,12 and sulfonated mesoporous silica,13,14 have been applied as heterogeneous catalysts for this reaction, but their catalytic reusability were not reported. Tatsumi and co-workers15 observed a steady catalytic reusability of Al-MCM-41 for rearrangement of cyclohexanone oxime; nevertheless, the conversion is at a low level (50.6%).Ionic liquids (ILs) featured with negligible volatility, high thermal stability, and alterable solubility have been extensively used as solvents/catalysts due to compositional and structural versatility.16 Acidic ILs task-specifically designed and prepared for catalyzing rearrangement of cyclohexanone oxime are in remarkable progress.17,18 Sulfonic acid1 and sulfonyl chloride19 have been incorporated to imidazolium-cation to result acid-functionalized IL catalysts, showing excellent yields to ε-caprolactam; however, in those systems, excess amounts of ILs with respect to the substrate oxime have to be added and the recovery of them are not as convenient as a heterogeneous solid catalyst. Moreover, Liu et al.18 developed a SO3H-tethered di-imidazolium IL catalyst that is recyclable for converting aromatic oximes, but its high activity can be observed only in the presence of the co-catalyst zinc chloride, the latter being harmful to environment by contaminating water. In the absence of ZnCl2, amide products can be obtained over the same catalyst.
Heteropolyacids (HPAs) are widely applied catalysts for numerous organic syntheses.20,21 Only very few HPA-derived catalysts have been tested in Beckmann rearrangements. The insoluble cesium salt of phosphotungstic acid is a well-known heterogeneous HPA catalyst for many acid-catalyzed reactions; however, as a recyclable catalyst for rearrangement of cyclohexanone oxime, it requires a higher temperature (150 °C) to reach the conversion of 80.8%.22 Notably in HPA chemistry, incorporation of organic units to HPAs has been an effective method to broaden utilizations of HPA-derived materials,23 wherein functionalized organic IL-cations are among the most effective modifiers to pair HPA-anions. Actually, some IL–HPA hybrid catalysts have been revealed as highly efficient and steadily reusable heterogeneous catalysts for organic syntheses.24–29 For example, sulfonated imidazolium is a previously reported acidic IL-cation,30 and our group finds that the sulfonated imidazolium salts of HPA behave as recyclable catalysts for esterifications.31–33 Very recently, we further observe that the sulfonated imidazolium salt of phosphotungstate [MIMPS]3PW12O40 can efficiently rearrange various oximes (including cyclohexanone oxime) to corresponding amides;34 nevertheless, the high yields to amides can only be obtained in the presence of ZnCl2. As a continuation of our early work, in this paper, we report the preparation and characterization of a IL–HPA hybrid material, dual-sulfonated 4,4′-dipyridinium phosphotungstate as the heterogeneous catalyst for low-temperature liquid-phase Beckmann rearrangement of cyclohexanone oxime. A high yield to ε-caprolactam is obtained without any co-catalysts like ZnCl2, together with the advantage of easy recovery and reuse of the solid catalyst. Various counterparts catalysts are also prepared and comparatively assessed in this rearrangement reaction.
2. Experiment
2.1 Synthesis of catalysts
For preparing N,N′-di(3-sulfopropyl) 4,4′-dipyridinium inner salt (DPySO3), 1,3-propane sulfone (20.5 mmol) was added into the ethanol solution of 4,4′-dipyridinium (10.0 mmol), and the mixture was heated to 80 °C under reflux for 24 h. Then, the resulting white precipitate was filtered, washed with diethyl ether repeatedly, and dried at 80 °C oven for 12 h to give the product DPySO3. Elemental analysis calcd: C, 47.99 wt%; N, 6.99 wt%; H, 5.03 wt%; S, 16.0 wt%. Found: C, 47.74 wt%; N, 6.77 wt%; H, 5.43 wt%; S, 15.33 wt%. 1H NMR (300 MHz, D2O, TMS); δ 2.54 (m, 4H, 2(–CH2)), 3.04 (t, 4H, 2(–CH2S)), 4.90 (t, 4H, 2(–CH2N)), 8.57 (d, 4H, 4(–CH)), 9.16 (d, 4H, 4(–CH)). 13C NMR (300 MHz, D2O, TMS); δ 28.9, 49.8, 62.9, 129.9, 145.9, 148.4. The major catalyst of this work N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphotungstate ([DPySO3H]1.5PW) was prepared consulting the procedure in our previous literature.31 In detail, aqueous solution of 12-phosphotungstic acid H3PW12O40 (H3PW) (1.0 mmol) was slowly added to the aqueous solution of DPySO3 (1.5 mmol) with vigorous stirring at room temperature for 24 h. Afterwards, water was removed in vacuum to give [DPySO3H]1.5PW as a solid, washed repeatedly with diethyl ether, and dried at 100 °C oven for 12 h to obtain the final white-yellow powder. Elemental analysis calcd: C, 8.27 wt%; N, 1.21 wt%; H, 0.95 wt%; S, 2.76 wt%. Found: C, 9.49 wt%; N, 1.23 wt%; H, 1.34 wt%; S, 2.88 wt%.Various control catalysts are prepared accordingly with minor modifications. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium silicotungstate ([DPySO3H]2SiW) was prepared by reacting DPySO3 with H4SiW12O40 (H4SiW) with the stoichiometric molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in aqueous solution. Elemental analysis calcd: C, 10.44 wt%; N, 1.52 wt%; H, 1.20 wt%; S, 3.48 wt%. Found: C, 11.68 wt%; N, 1.52 wt%; H, 1.54 wt%; S, 3.63 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphomolybdate ([DPySO3H]1.5PMo) was prepared by reacting DPySO3 with H3PMo12O40 (H3PMo) with the stoichiometric molar ratio 1.5
1 in aqueous solution. Elemental analysis calcd: C, 10.44 wt%; N, 1.52 wt%; H, 1.20 wt%; S, 3.48 wt%. Found: C, 11.68 wt%; N, 1.52 wt%; H, 1.54 wt%; S, 3.63 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphomolybdate ([DPySO3H]1.5PMo) was prepared by reacting DPySO3 with H3PMo12O40 (H3PMo) with the stoichiometric molar ratio 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in aqueous solution. Elemental analysis calcd: C, 11.88 wt%; N, 1.73 wt%; H, 1.36 wt%; S, 3.96 wt%. Found: C, 12.59 wt%; N, 1.72 wt%; H, 1.62 wt%; S, 3.96 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphotungstate ([DPySO3H]HPW) was prepared by reacting DPySO3 with H3PW with the stoichiometric molar ratio 1
1 in aqueous solution. Elemental analysis calcd: C, 11.88 wt%; N, 1.73 wt%; H, 1.36 wt%; S, 3.96 wt%. Found: C, 12.59 wt%; N, 1.72 wt%; H, 1.62 wt%; S, 3.96 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphotungstate ([DPySO3H]HPW) was prepared by reacting DPySO3 with H3PW with the stoichiometric molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in aqueous solution. Elemental analysis calcd: C, 5.85 wt%; N, 0.854 wt%; H, 0.70 wt%; S, 1.95 wt%. Found: C, 6.55 wt%; N, 0.85 wt%; H, 1.24 wt%; S, 2.23 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium silicotungstate ([DPySO3H]H2SiW) was prepared by reacting DPySO3 with H4SiW with the stoichiometric molar ratio 1
1 in aqueous solution. Elemental analysis calcd: C, 5.85 wt%; N, 0.854 wt%; H, 0.70 wt%; S, 1.95 wt%. Found: C, 6.55 wt%; N, 0.85 wt%; H, 1.24 wt%; S, 2.23 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium silicotungstate ([DPySO3H]H2SiW) was prepared by reacting DPySO3 with H4SiW with the stoichiometric molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in aqueous solution. Elemental analysis calcd: C, 5.86 wt%; N, 0.854 wt%; H, 0.73 wt%; S, 1.95 wt%. Found: C, 6.51 wt%; N, 0.81 wt%; H, 1.35 wt%; S, 1.91 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphomolybdate ([DPySO3H]HPMo) was prepared by reacting DPySO3 with H3PMo with the stoichiometric molar ratio 1
1 in aqueous solution. Elemental analysis calcd: C, 5.86 wt%; N, 0.854 wt%; H, 0.73 wt%; S, 1.95 wt%. Found: C, 6.51 wt%; N, 0.81 wt%; H, 1.35 wt%; S, 1.91 wt%. N,N′-di(3-sulfopropyl) 4,4′-dipyridinium phosphomolybdate ([DPySO3H]HPMo) was prepared by reacting DPySO3 with H3PMo with the stoichiometric molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in aqueous solution. Elemental analysis calcd: C, 8.63 wt%; N, 1.258 wt%; H, 1.03 wt%; S, 2.876 wt%. Found: C, 9.14 wt%; N, 1.20 wt%; H, 1.35 wt%; S, 2.79 wt%.
1 in aqueous solution. Elemental analysis calcd: C, 8.63 wt%; N, 1.258 wt%; H, 1.03 wt%; S, 2.876 wt%. Found: C, 9.14 wt%; N, 1.20 wt%; H, 1.35 wt%; S, 2.79 wt%.
2.2 Characterizations
All chemicals were of analytical grade and used as received. FT-IR spectra for samples in KBr disks were recorded on Nexus 870 FT-IR spectrometer. 1H NMR spectra were measured with a Brüker DPX 300 spectrometer at ambient temperature in D2O using TMS as internal reference. Elemental analyses (C, H, N and S) were performed on a CHNS elemental analyzer (FlashEA 1112). TG analysis was carried out with a STA409 instrument in dry air at a heating rate of 10 °C min−1.2.3 Catalytic tests
Beckmann rearrangement of cyclohexanone oxime was carried out in a 25 ml round-bottomed flask equipped with a reflux condenser. Typically, the solvent of benzonitrile (PhCN) (4 ml), cyclohexanone oxime (1.0 mmol) and catalyst (0.1 mmol) were charged into the flask reactor successively to make a reaction mixture, which was heated at 130 °C for 2 h. Catalyst amount, solvent type, reaction temperature and time were changed to investigate the influences of reaction conditions. At the end of the reaction the resulting product mixture was cooled down and the liquid phase was decanted for analysis. Quantitative analyses were conducted with gas chromatography (Shimadzu GC-2014) equipped with a FID detector and a capillary column (SE-30; 50 m × 0.25 mm × 0.3 μm). Tetradecane was added as the internal standard for GC analysis. Only cyclohexanone derived from the deoximation of cyclohexanone oxime was detected as the by-product besides the major rearrangement product ε-caprolactam. Conversion of cyclohexanone oxime (conv.%), selectivity to ε-caprolactam (sel.%) and yield to ε-caprolactam (Y%) are calculated as the following: conv.% = (mol ε-caprolactam + mol cyclohexanone)/mol initial cyclohexanone oxime; sel.% = mol ε-caprolactam/(mol ε-caprolactam + mol cyclohexanone); Y% = conv.% × sel.%.3. Results and discussion
3.1 Characterization of the catalyst [DPySO3H]1.5PW
The TG result for [DPySO3H]1.5PW in Fig. 1A demonstrates a stable structure up to 250 °C. The slight weight loss at the early heating stage before 150 °C is due to the release of moisture and constitutional water, while the drastic weight loss above 250 °C arises from the complete decomposition of the organic moiety combined with the collapse of the inorganic Keggin structure forming P2O5 and WO3. In the later range, the weight loss of ca. 6.8 wt% in 150–375 °C is probably due to the decomposition of 4,4′-dipyridinium framework, and that of ca. 10.8 wt% in 375–620 °C may attribute to the decomposition of the remaining organic moiety. Collapse of inorganic moiety in this high temperature range cannot cause a weight change. The total weight loss in the range of 150–620 °C was ca. 17.6 wt%, very close to the theoretical data 17.3 wt% calculated according to the chemical composition of [DPySO3H]1.5PW.Fig. 1B illustrates the XRD patterns of [DPySO3H]1.5PW and the parent sample H3PW. Neat H3PW displayed a set of well resolved sharp diffraction peaks for the crystal structure of the Keggin HPA. Disappearance of these peaks on [DPySO3H]1.5PW demonstrates destroying of the long-range order of H3PW crystal lattice due to the self-assembly of HPA-anions with large sulfonated 4,4′-bipyridinium cations.24 The emerging of the broad and weak reflections at low angles of ca. 5.5° and 8.2° indicates that the non-crystal hybrid [DPySO3H]1.5PW involves regular ion-pair arrays.34
FT-IR spectra of H3PW12O40 and [DPySO3H]1.5PW are compared in Fig. 2. The four bands for neat H3PW at 1080, 982, 890 and 802 cm−1 are assigned to νas(P–O), νas(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) (terminal), νas(W–Ob–W) (corner-sharing) and νas(W–Oc–W) (edge-sharing), respectively, consistent to the Keggin structure of HPAs. For [DPySO3H]1.5PW, the above four peaks appeared obviously, evidencing that Keggin structure is retained after the counter protons of H3PW are replaced by the IL-dication [DPySO3H]2+. Besides, characteristic vibrations for organic moiety were well detectable. For instances, the band at 1560 cm−1 is assigned to C
O) (terminal), νas(W–Ob–W) (corner-sharing) and νas(W–Oc–W) (edge-sharing), respectively, consistent to the Keggin structure of HPAs. For [DPySO3H]1.5PW, the above four peaks appeared obviously, evidencing that Keggin structure is retained after the counter protons of H3PW are replaced by the IL-dication [DPySO3H]2+. Besides, characteristic vibrations for organic moiety were well detectable. For instances, the band at 1560 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration on pyridinium rings, and 1446 cm−1 is due to the –CH2 bending vibration. Moreover, the bands at 1230 and 1174 cm−1 are respectively attributed to νas(SO2) and νs(SO2) in sulfonic group. These observations confirm the structure of the hybrid [DPySO3H]1.5PW via ionic linkage of the dual-sulfonated dipyridinium with the Keggin 12-phosphotungstic anion.33
N stretching vibration on pyridinium rings, and 1446 cm−1 is due to the –CH2 bending vibration. Moreover, the bands at 1230 and 1174 cm−1 are respectively attributed to νas(SO2) and νs(SO2) in sulfonic group. These observations confirm the structure of the hybrid [DPySO3H]1.5PW via ionic linkage of the dual-sulfonated dipyridinium with the Keggin 12-phosphotungstic anion.33
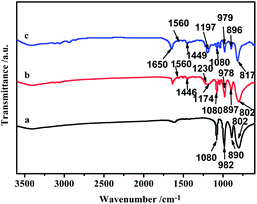 | ||
| Fig. 2 FT-IR spectra of selected catalysts (a) H3PW12O40, (b) fresh [DPySO3H]1.5PW, (c) recovered [DPySO3H]1.5PW from the fourth recycling run. | ||
3.2 Beckmann rearrangement of cyclohexanone oxime over various catalysts
The results of the Beckmann rearrangement of cyclohexanone oxime catalyzed by [DPySO3H]1.5PW and various control samples are listed in Table 1. The reaction did not proceed without a catalyst (entry 1). The parent HPA catalyst H3PW offered a high conversion of cyclohexanone oxime 99% with considerable selectivity to ε-caprolactam 65% (entry 2); however, H3PW has difficulty in catalyst isolation due to its homogeneous nature. It is interesting to see that [DPySO3H]1.5PW not only caused a liquid–solid biphasic reaction, but also showed a perfect conversion 100% with enhanced selectivity 73% (entry 3). The by-product detected was cyclohexanone generated from the side-reaction of the hydrolysis of cyclohexanone oxime, while other possible by-products like cyclohexen-1-one, nitriles and dimers were in trace amount. In our previous report, mono-sulfonated imidazolium counterpart [MIMPS]3PW alone presented an extremely low conversion 22% with a low selectivity 45% (entry 5), while the addition of co-catalyst ZnCl2 enhanced both conversion (100%) and selectivity (83%) (entry 6).35 In contrast, when ZnCl2 was to combine [DPySO3H]1.5PW, the selectivity increased from 73% to 80% (entry 4), which is unremarkable compared to [MIMPS]3PW (from 45% to 83%). Therefore, the high activity obtained in this work is advantageous for [DPySO3H]1.5PW, considering the needless of adding the non-reusable and environmental harmful ZnCl2.| Entry | Catalyst | Conv./% | Sel./% | Y/% |
|---|---|---|---|---|
| a Reaction conditions: 1.0 mmol cyclohexanone oxime, 10 mol% catalyst (0.1 mmol for entries 2–4, 8, 9 and 11; 0.075 mmol for entries 7 and 10), 4 ml PhCN, 130 °C, 2 h.b Adding 15 mol% ZnCl2 (0.15 mmol).c From ref. 35 with reaction conditions for entry 5: 2 mmol cyclohexanone oxime, 10 mol% catalyst (0.2 mmol), 5 ml MeCN, 90 °C, 1 h; for entry 6: adding 30 mol% ZnCl2 (0.6 mmol) based on entry 5. | ||||
| 1 | None | 0 | — | 0 |
| 2 | H3PW | 99 | 65 | 64 |
| 3 | [DPySO3H]1.5PW | 100 | 73 | 73 |
| 4b | [DPySO3H]1.5PW | 100 | 80 | 80 |
| 5c | [MIMPS]3PW | 22 | 45 | 9.9 |
| 6c | [MIMPS]3PW | 100 | 83 | 83 |
| 7 | [DPySO3H]2SiW | 84 | 64 | 54 |
| 8 | [DPySO3H]1.5PMo | 100 | 39 | 39 |
| 9 | [DPySO3H]HPW | 96 | 67 | 65 |
| 10 | [DPySO3H]H2SiW | 67 | 69 | 46 |
| 11 | [DPySO3H]HPMo | 100 | 18 | 18 |
In previous works on heterogeneous Beckmann rearrangement of cyclohexanone oxime, Wang et al.13 obtained a conversion 51.9% with selectivity 81.3% (yield 42.2%) over the mesoporous silica-supported acid catalyst, and Mitsudome et al.36 achieved a yield to ε-caprolactam 74% at a longer time (20 h) with the catalyst of titanium cation-exchanged montmorillonite. Compared to them, the reactivity of rearrangement of cyclohexanone oxime with full consumption of substrate and 73% selectivity (yield 73%) on the present catalyst [DPySO3H]1.5PW is relativity higher. For interpreting the catalytic performance of [DPySO3H]1.5PW, control catalysts were prepared and evaluated under same conditions (Table 1, entries 7–11). Besides PW, the two commonly seen Keggin HPA-anions, SiW and PMo, were also respectively paired to the dual-sulfonated 4,4′-dipyridinium. The resulting [DPySO3H]2SiW and [DPySO3H]1.5PMo showed much lower conversion and/or selectivity than [DPySO3H]1.5PW, with [DPySO3H]1.5PMo giving the lowest yield (entries 7 and 8). The catalytic activities of the three dual-sulfonated IL–HPA catalysts based on yields to ε-caprolactam are in sequence of [DPySO3H]1.5PW > [DPySO3H]2SiW > [DPySO3H]1.5PMo, which consists to the order of the acid strength for their parent HPAs: H3PW > H4SiW > H3PMo.37,38 It is known that the acidities of the counter protons in parent HPAs are associated with the electron-withdrawing capability of the Keggin-structured-anion frameworks.37 It is thus proposed for the three IL–HPA hybrids that the acidities of sulfonic groups tethered to 4,4′-dipyridinium cations should be tuned and enhanced by the ionic linked HPA-anions that are electron-withdrawing in nature. This may be a reason for the activity sequence of [DPySO3H]1.5PW > [DPySO3H]2SiW > [DPySO3H]1.5PMo, as well as the higher activity of [DPySO3H]1.5PW than its parent H3PW. Another obvious indication of the above proposal is the similar activity order for the partially proton-substituted hybrids [DPySO3H]HPW > [DPySO3H]H2SiW > [DPySO3H]HPMo (entries 9–11). Further, it is seen in Table 1 that the partial proton-substituted counterparts [DPySO3H]HPW, [DPySO3H]H2SiW and [DPySO3H]HPMo gave lower yields than [DPySO3H]1.5PW, [DPySO3H]2SiW and [DPySO3H]1.5PMo respectively. The result implies that the –SO3H related acidic protons play a more significant role in catalyzing Beckmann rearrangement of cyclohexanone oxime than the remaining protons on HPAs.
3.3 Influence of reaction conditions for [DPySO3H]1.5PW-catalyzed Beckmann rearrangement
The influence of solvents on [DPySO3H]1.5PW-catalyzed Beckmann rearrangement of cyclohexanone oxime is listed in Table 2. The solvents used include benzonitrile (PhCN), toluene, dimethylsulfoxide (DMSO), dimethyl formamide (DMF), acetonitrile (MeCN), phenylacetonitrile, o-tolunitrile and ethyl acetate. The results showed that PhCN is the most suitable solvent for the present catalyst as it gave the highest yield to ε-caprolactam 73% (entry 1). The fact that PhCN behaves as an excellent solvent for this reaction has been observed before.15,22 The polarity and basicity of a solvent are key parameters for catalytic transformation of oximes to corresponding amides on acidic active sites.15 It is thus suggested that PhCN with optimum medium polarity and basicity must have played importance roles in balancing the competitive adsorption with the basic substrate, and the followed desorption with the basic amide produced on acid active sites.22 Similar to PhCN in molecular structure, phenylacetonitrile and o-tolunitrile gave considerable yields of 43.7% and 62.5%, respectively (entries 2 and 3), though lower compared to PhCN (73%). On the contrary, the apolar toluene resulted in a liquid–liquid–solid triphasic reaction with severe mass transfer problem, and was almost inactive (entry 4).35 Also, ethyl acetate with very weak polarity and basicity showed extremely low conversion (entry 5). However, the two polar solvents DMF and DMSO presented no amide product (entries 6 and 8), mostly due to their stronger basicity that may cause strong adsorption on catalyst acid sites and thus hinder accesses of substrates to active sites.13,15 It is interesting to note that no amide product was detected in DMF or DMSO even aided with ZnCl2 (entries 7 and 9), further indicating that ZnCl2 cannot be a cocatalyst with [DPySO3H]1.5PW as already shown in Table 2. The fact that in DMSO oxime hydrolysis became the main route with a perfect conversion is not understandable at this moment. Finally, MeCN is a well-known effective solvent for ZnCl2-cocatalyzed rearrangements,18,35 but it did not work well with the catalyst [DPySO3H]1.5PW (entry 10). MeCN is a low melting point solvent, providing a reflux condition at 82 °C, which is lower than 130 °C for PhCN, it is therefore understandable that for the present non-ZnCl2-aided catalyst it is almost inactive.| Entry | Solvent | Conv./% | Sel./% | Y/% |
|---|---|---|---|---|
| a Reaction conditions: 1.0 mmol cyclohexanone oxime, 10 mol% catalyst (0.1 mmol), 4 ml solvent, 130 °C, 2 h.b 110 °C.c Adding 15 mol% ZnCl2 (0.15 mmol).d Adding 30 mol% ZnCl2 (0.3 mmol).e 82 °C. | ||||
| 1 | PhCN | 100 | 73 | 73 |
| 2 | Phenylacetonitrile | 75 | 58.3 | 43.7 |
| 3 | o-Tolunitrile | 100 | 62.5 | 62.5 |
| 4b | Toluene | 1.3 | 0 | 0 |
| 5 | Ethyl acetate | 2.4 | 0 | 0 |
| 6 | DMSO | 100 | 0 | 0 |
| 7c | DMSO | 100 | 0 | 0 |
| 8 | DMF | 4.8 | 0 | 0 |
| 9d | DMF | 12 | 0 | 0 |
| 10e | MeCN | 2.6 | 0 | 0 |
Fig. 3 shows the effect of the amount of catalyst [DPySO3H]1.5PW on the rearrangement of cyclohexanone oxime with PhCN as the solvent. When the molar ratio of [DPySO3H]1.5PW to cyclohexanone oxime increased from 1% to 3%, both conversion and selectivity increased quickly (conversion from 30% to 66%, and selectivity from 57% to 72%). Further increase of the catalyst amount to 10 mol% led to the complete consumption of substrate (100% conversion) with selectivity maintained at 73%. Therefore, 10 mol% is the suitable catalyst amount.
 | ||
| Fig. 3 Effect of catalyst amount on Beckmann rearrangement of cyclohexanone oxime over [DPySO3H]1.5PW (reaction conditions: 1.0 mmol cyclohexanone oxime, 4 ml PhCN, 130 °C, 2 h). | ||
Fig. 4 displays the influence of reaction temperature on [DPySO3H]1.5PW-catalyzed rearrangement of cyclohexanone oxime with PhCN as the solvent. It is seen that the raise of the reaction temperature is propitious for improving conversion and selectivity. The conversion increased dramatically from 13% at 90 °C to full conversion at 130 °C, with the selectivity enhanced simultaneously from 33% to 73%. This change may be attributed to the endothermic nature of Beckmann rearrangement reaction, which favors the conversion of oxime to amide at increased temperatures.39
 | ||
| Fig. 4 Effect of reaction temperature on Beckmann rearrangement of cyclohexanone oxime over [DPySO3H]1.5PW (reaction condition: 1.0 mmol cyclohexanone oxime, 0.1 mmol catalyst, 4 ml PhCN, 2 h). | ||
Fig. 5 depicts the impact of reaction time on the rearrangement of cyclohexanone oxime with PhCN as the solvent. The reaction catalyzed by [DPySO3H]1.5PW took place quickly with the obtaining of 88% conversion and ca. 60% selectivity to amide within 30 min. Then the reaction rate decreased with prolonging the reaction time. The conversion of cyclohexanone oxime gradually increased to 100% as the reaction proceeded up to 2 h with 73% selectivity. At the reaction time of 2.5 h, the selectivity of amide was maintained at around 70%, indicating a steady state of the reaction. Thus the optimal reaction time is 2 h for the [DPySO3H]1.5PW-catalyzed rearrangement.
 | ||
| Fig. 5 Effect of reaction time on Beckmann rearrangement of cyclohexanone oxime over [DPySO3H]1.5PW (reaction condition: 1.0 mmol cyclohexanone oxime, 0.1 mmol catalyst, 4 ml PhCN, 130 °C). | ||
3.4 Recycling test for [DPySO3H]1.5PW catalyst in Beckmann rearrangement
After reaction, the powdered catalyst [DPySO3H]1.5PW could be recovered by filtration from product mixture, and thus a four-run recycling test for [DPySO3H]1.5PW was carried out under the optimized conditions. As shown in Fig. 6, a slow decrease of conversion was observed without much loss of selectivity. At the fourth run, it still gave 70% conversion and 70% selectivity. TOF [turnover frequency: ε-caprolactam (mol) produced on sulfoacid site (per mole and per hour)] is calculated based on the amount of catalyst recovered [1st run (fresh) 0.1 mmol, 2nd run 0.088 mmol, 3rd run 0.079 mmol, 4th run 0.068 mmol], which is also plotted in Fig. 6. It can be seen that no significant decrease in TOF was observed, seemingly implying that the capability of each active site in the recovered catalyst for converting cyclohexanone oxime to ε-caprolactam is relatively stable within the recycling test duration. However, this does not mean that the catalyst is absolutely stable in recycling. When we compare the 3rd run (recovered catalyst amount: 0.079 mmol) with one of the data in Fig. 3 (fresh catalyst amount: 0.08 mmol), it is found that the obtained 80% conversion with 70% selectivity over the recovered catalyst is still lower than the 89% conversion with 74% selectivity over the fresh catalyst, which clearly indicates the slow deactivation of the present catalyst.Improving catalytic reusability of a heterogeneous catalyst for acid-catalyzed rearrangement of cyclohexanone oxime is a very difficult issue, because the strong basicity of oxime substrate and/or amide product would almost inevitably contaminate the acid active centers of a solid catalyst,19 such that many previous studies did not deal with the reuse of their catalysts.13,14,40 Our early work observed a drastic decrease of activity over the recovered IL-derived catalyst [MIMPS]3PW12O40 (in the third run, 53% of its original activity was retained).35 Moreover, that recycling data was obtained in the presence of the freshly added ZnCl2 for each recycling run. Clearly, the reusability of the catalyst [DPySO3H]1.5PW in this work (in the third run, 80% of its original activity was retained) is greatly improved compared with the early work. In the FT-IR spectrum for the recovered [DPySO3H]1.5PW from the fourth recycling run (Fig. 2), one can see the well retaining of the signals for the Keggin structure of phosphotungstate anion. However, the characteristic vibrations for the product molecule ε-caprolactam occurred as well; the band at 1650 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which is shifted from the original position 1679 cm−1 due to the interaction of the acidic –SO3H with the basic amide product and/or oxime substrate.19,35 The characterization result thus demonstrates that the deactivation of the present catalyst [DPySO3H]1.5PW, though much lower than previous work, is mostly due to the contamination of catalyst acid sites by the basic amide product and/or oxime substrate.
O, which is shifted from the original position 1679 cm−1 due to the interaction of the acidic –SO3H with the basic amide product and/or oxime substrate.19,35 The characterization result thus demonstrates that the deactivation of the present catalyst [DPySO3H]1.5PW, though much lower than previous work, is mostly due to the contamination of catalyst acid sites by the basic amide product and/or oxime substrate.
4. Conclusions
In this work, the dual-sulfoacid-tethered 4,4′-dipyridinium phosphotungstate [DPySO3H]1.5PW12O40 is task-specifically developed for liquid-phase biphasic heterogeneous Beckmann rearrangement of cyclohexanone oxime, showing highly efficient activity and greatly improved reusability. In the absence of the environmental harmful cocatalyst ZnCl2, 10 mol% catalyst [DPyPSO3H]1.5PW12O40 alone well converts cyclohexanone oxime to ε-caprolactam (100% conversion and 73.0% selectivity) within 2 h at 130 °C. After reaction, the powder catalyst [DPySO3H]1.5PW can be recovered easily via filtration, presenting a slow decrease of conversion but still with a considerably high activity after four recycling runs of rearrangement reaction. Though further improvement of catalyst reusability is still needed, the present results make a clear progress in recyclable heterogeneous catalysts for liquid-phase Beckmann rearrangement of cyclohexanone oxime.Acknowledgements
We thank the National Natural Science Foundation of China (no. 21136005 and 21303084), and Jiangsu Provincial Natural Science Foundation for Youths (no. SBK201342704).Notes and references
- R. Turgis, J. Estager, M. Draye, V. Ragaini, W. Bonrath and J. MLeveque, ChemSusChem, 2010, 3, 1403 CrossRef CAS PubMed
.
- G. Bellussi and C. Perego, CATTECH, 2000, 4, 4 CrossRef CAS
.
- M. Anilkumar and W. F. Holderich, J. Catal., 2008, 260, 17 CrossRef CAS
.
- D. S. Zhang, R. J. Wang and X. X. Yang, Catal. Commun., 2011, 12, 399 CrossRef CAS
.
- A. Cesana, S. Palmery and R. Buzzoni, Catal. Today, 2010, 154, 264 CrossRef CAS
.
- W. C. Li, A. H. Lu, R. Palkovits, W. Schmidt, B. Spliethoff and F. Schuth, J. Am. Chem. Soc., 2005, 127, 12595 CrossRef CAS PubMed
.
- Y. J. Zhang, Y. Q. Wang, Y. F. Bu, Z. T. Mi and W. Wu, Catal. Commun., 2005, 6, 53 CrossRef CAS
.
- V. Srivastava, R. Patel, G. Yadav and L. Yadav, Chem. Commun., 2010, 46, 5808 RSC
.
- Y. Furuya, K. Ishihara and H. Yamamoto, J. Am. Chem. Soc., 2005, 127, 11240 CrossRef CAS PubMed
.
- M. Zhu, C. T. Cha, W. P. Deng and X. X. Shi, Tetrahedron Lett., 2006, 47, 4861 CrossRef CAS
.
- Y. M. Chung and H. K. Rhee, J. Mol. Catal. A: Chem., 2001, 175, 249 CrossRef CAS
.
- N. R. Shiju, M. AnilKumar, W. F. Hoelderich and D. R. Brown, J. Phys. Chem. C, 2009, 113, 7735 CAS
.
- X. Wang, C. Chen, S. Chen, Y. Mou and S. Cheng, Appl. Catal., A, 2005, 281, 47 CrossRef CAS
.
- W. Zhao, P. Salame, V. Herledan, F. Launay, A. Gédéon and T. Qi, J. Porous Mater., 2010, 17, 335 CrossRef
.
- C. Ngamcharussrivichai, P. Wu and T. Tatsumi, J. Catal., 2004, 227, 448 CrossRef CAS
.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed
.
- S. Guo, Z. Du, S. Zhang, D. Li, Z. Li and Y. Deng, Green Chem., 2006, 8, 296 RSC
.
- X. Liu, L. Xiao, H. Wu, Z. Li, J. Chen and C. Xia, Catal. Commun., 2009, 10, 424 CrossRef CAS
.
- Z. Du, Z. Lia, Y. Gua, J. Zhang and Y. Deng, J. Mol. Catal. A: Chem., 2005, 237, 80 CrossRef CAS
.
- T. Okuhara, Catal. Today, 2002, 73, 167 CrossRef CAS
.
- F. Chai, F. Cao, F. Zhai, Y. Chen, X. Wang and Z. Su, Adv. Synth. Catal., 2007, 349, 1057 CrossRef CAS
.
- N. Shiju, H. Williams and D. Brown, Appl. Catal., B, 2009, 90, 451 CrossRef CAS
.
- A. Dolbecq, E. Dumas, C. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed
.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao and J. Huang, Green Chem., 2011, 13, 1636 RSC
.
- P. Zhao, Y. Leng, M. Zhang, J. Wang, Y. Wu and J. Huang, Chem. Commun., 2012, 48, 5721 RSC
.
- P. Zhao, Y. Leng and J. Wang, Chem. Eng. J., 2012, 204, 72 CrossRef
.
- Y. Leng, J. Wang, D. Zhu, L. Shen, P. Zhao and M. Zhang, Chem. Eng. J., 2011, 173, 620 CrossRef CAS
.
- W. Zhang, Y. Leng, P. Zhao, J. Wang, D. Zhu and J. Huang, Green Chem., 2011, 13, 832 RSC
.
- P. Zhao, M. Zhang, Y. Wu and J. Wang, Ind. Eng. Chem. Res., 2012, 51, 6641 CrossRef CAS
.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Jr Davis, J. Am. Chem. Soc., 2002, 124, 5962 CrossRef CAS PubMed
.
- Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge and L. Shen, Angew. Chem., Int. Ed., 2009, 48, 168 CrossRef CAS PubMed
.
- W. Zhang, Y. Leng, D. Zhu, Y. Wu and J. Wang, Catal. Commun., 2009, 11, 151 CrossRef CAS
.
- Y. Leng, J. Wang, D. Zhu, Y. Wu and P. Zhao, J. Mol. Catal. A: Chem., 2009, 313, 1 CrossRef CAS
.
- X. Yan, P. Zhu, J. Fei and J. Li, Adv. Mater., 2010, 22, 1283 CrossRef CAS PubMed
.
- X. Zhang, D. Mao, Y. Leng, Y. Zhou and J. Wang, Catal. Lett., 2013, 143, 193 CrossRef CAS
.
- T. Mitsudome, T. Matsuno, S. Sueoka, T. Mizugaki, K. Jitsukawa and K. Kaneda, Tetrahedron Lett., 2012, 53, 5211 CrossRef CAS
.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed
.
- J. K. Kim, J. Choi, J. Song, J. Yi and I. Song, Catal. Commun., 2012, 27, 5 CrossRef CAS
.
- K. You, L. Mao, D. Yin, P. Liu and H. Luo, Catal. Commun., 2008, 9, 1521 CrossRef CAS
.
- C. Ngamcharussrivichai, P. Wu and T. Tatsumi, Appl. Catal., A, 2005, 288, 158 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2014 |