Photobase generating monomers: synthesis, evaluation and utilization for fabricating fluorescence patterns
Lanlan Liu,
Jinbao Guo,
Zihao Li and
Jie Wei*
College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: weij@mail.buct.edu.cn; Fax: +86-10-64427628; Tel: +86-10-64454598
First published on 6th March 2014
Abstract
In this study, we fabricated four new photobase generating monomers, in which carbamate units functioned as the photobase generating moieties, methacrylate units served as the polymerizable groups and four different groups, succinimido, camphorquinone 3-oximino, di-2-thienyl ketoximino and difuril dioximino units, acted as photosensitive species. The effect of different photosensitive species on the photochemical behavior of the monomers was investigated in detail. The results demonstrate that, among the four monomers, succinimido methacryloxyethyl carbamate (SMC) exhibits the most stable photobase generating property. Furthermore, a copolymer containing SMC is used for a fluorescence imaging material. The fluorescence spectrum of the fluorescamine-treated irradiated copolymer film showed a strong fluorescence in the range of 420–650 nm with a fluorescence maximum at 476 nm. The patterns in the copolymer films exhibit very distinct fluorescence images with colors of blue, green and red at certain observation wavelengths.
1. Introduction
In recent years, the formation of fluorescent images in thin polymer films has attracted considerable interest due to their potential application in the areas of displays,1,2 optical memory devices3 and molecular switches,4 as well as in the sensor5,6 and imaging industries.7 A variety of techniques exist for creating patterned fluorescence images on solid substrates, including photoirradiation,8 micro-contact printing,9 inkjet/screen printing10 and vapor deposition.11,12 Among these methods, the photoirradiation technique represents the most popular and practical approach for fluorescence image generation. The fluorescence imaging in polymer films with this technique can be effectively manipulated by photochromism,4a,4b,7a,13 photoinduced polymerization,1,14 photoinduced oxidative degradation or molecular orientation,15 photoacid induced protonation,3a,3b,16 and the use of a prefluorescent radical probe.17Bases originating from photobase generating systems upon irradiation18 are being increasingly used in the development of new technologies. For example, this system has been applied to the photolithographic micropatterning of electroluminescent polymers19 and the patterning of conductive polyaniline films,20 as well as various types of photoresists.21 However, there are few reports on photobase-generating systems for the formation of fluorescence patterns compared with photo-radical and photoacid generators. Won et al.22 reported a fluorescence imaging process based on a polymeric photobase generator containing oxime–urethane groups through the use of fluorescamine, in which fluorescamine enabled the sensitive fluorometric determination of primary amines and amino acids. Eun and collaborators1 constructed a film emitting red, green, and blue fluorescence selectively, in which green-fluorescent fluorescein molecules were encapsulated in red-fluorescent PDA vesicles, and then a blue-fluorescent component was introduced through the reaction between the terminal amine groups on the PDA vesicle surfaces and fluorescamine. Kyu and co-workers23 used a polymer film containing phthalimido carbamate groups as a bicolor fluorescent imaging material. The polymer film was irradiated with 254 nm UV light through a photomask and then treated with fluorescamine and rhodamine B consecutively. Various colors of fluorescent micropatterns – green, red, or red-yellow – were obtained on a single polymer film by varying the excitation wavelength. Furthermore, Kyu et al.24 also reported that irradiation of a polymer film bearing anilide groups led to photo-anilide rearrangement to form aromatic amino groups in the irradiated area; then, after reacting with fluorescamine, a fluorescent micropattern was formed. Although many reports have focused on different approaches with the aim of fabricating fluorescent images using photobase generators, there are only a few on the study of improving the photosensitivity of the photosensitive groups.23
In this study, we fabricated four novel photobase generating monomers, including succinimido methacryloxyethyl carbamate (SMC), camphorquinone 3-oximino methacryloxyethyl carbamate (ACMC), di-2-thienyl ketoximino methacryloxyethyl carbamate (TKMC) and difuril dioximino methacryloxyethyl carbamate (DFMC), in which carbamate units acted as the photobase generating moieties and methacrylate units served as the polymerizable groups. Herein, we used four kinds of groups, succinimido, camphorquinone 3-oximino, di-2-thienyl ketoximino and difuril dioximino, as photosensitive species which were different from the widely investigated benzophenoneoxime22 and phthalimido23 species. The influence of photosensitive moieties on the photochemical characteristics of the four monomers were thoroughly addressed by observing their changes in UV-Vis absorption spectra, FT-IR spectra, fluorescence spectra after treatment with fluorescamine and their changes of pH values. Based on the above studies, SMC was chosen to synthesize a copolymer for the preparation of multi-color fluorescence patterns. The fluorescence images obtained in this experiment show blue, green and red fluorescence patterns depending on the observation wavelengths. This copolymer material is of potential interest in the preparation of fluorescent images for photonic and optical applications.
2. Experimental
2.1 Materials
N-Hydroxysuccinimide (98%) was purchased from Heowns Biochem Technologies. Camphorquinone 3-oxime (99%) and 2-isocyanatoethyl methacrylate (98%) were bought from Tokyo Chemical Industry Co. Ltd. Bis(2-thienyl) ketoxime (97%), 2,2′-azo-bis(isobutyronitrile) (AIBN, 99%) and α-furil dioxime (99%) were obtained from Alfa Aesar. Fluorescamine (98%) and 1-methoxy-2-propyl acetate (PMA, AR) were purchased from J&K Scientific Co. Ltd. Triethylamine (TEA, AR), methyl methacrylate (MMA), acetone (AR), cyclohexane (AR) and methanol (AR) were supplied by Beijing Chemical Works, and were all used as received. Tetrahydrofuran (THF, anhydrous, AR) was purchased from Heowns Biochem Technologies and stored in 4 Å molecular sieves. Analytical thin layer chromatography (TLC) was performed on commercially coated 60 mesh GF254 glass plates.2.2 Synthesis of photobase generating monomers
The general procedure for the synthesis of the four photobase generating monomers is similar to that of the previous report,22 here we present a simple description. To a solution of R–OH (5 mmol) and TEA (0.03 mL) in THF (50 mL), 2-isocyanatoethyl methacrylate (5.2 mmol) in THF (10 mL) was added dropwise with stirring at room temperature for 12 h. R represents four different photosensitive groups as described in Scheme 1. After the completion of the reaction, the solvent was removed under reduced pressure at 40 °C. Crude products were recrystallized from methanol. | ||
| Scheme 1 Reaction process for the synthesis of photobase generating monomers used in this experiment along with their chemical structures. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1636 (C
O stretching), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 stretching), 1510 (C–H asym stretching), 1296, 1217 (C–O–C asym stretching), 1101 (C–O–C sym stretching), 941 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.47 (t, J = 5.62 Hz, 1H, NH), 6.08 (s, 1H, C
CH2 stretching), 1510 (C–H asym stretching), 1296, 1217 (C–O–C asym stretching), 1101 (C–O–C sym stretching), 941 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.47 (t, J = 5.62 Hz, 1H, NH), 6.08 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.69 (s, 1H, C
CH2), 5.69 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.14 (t, J = 5.26 Hz, 2H, CH2–CH2), 3.40 (m, 2H, CH2–CH2), 2.77 (m, 4H), 1.89 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 170.76, 166.46, 152.03, 135.58, 126.17, 62.86, 40.01, 25.23, 17.89.
CH2), 4.14 (t, J = 5.26 Hz, 2H, CH2–CH2), 3.40 (m, 2H, CH2–CH2), 2.77 (m, 4H), 1.89 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 170.76, 166.46, 152.03, 135.58, 126.17, 62.86, 40.01, 25.23, 17.89.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1642 (C
O stretching), 1642 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1544 (C–H asym stretching), 1316, 1294, 1250 (C–O–C asym stretching), 1167, 1104 (C–O–C sym stretching), 965 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 7.79 (t, J = 5.64 Hz, 1H, NH), 6.06 (s, 1H, C
C stretching), 1544 (C–H asym stretching), 1316, 1294, 1250 (C–O–C asym stretching), 1167, 1104 (C–O–C sym stretching), 965 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 7.79 (t, J = 5.64 Hz, 1H, NH), 6.06 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.69 (s, 1H, C
CH2), 5.69 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.16 (t, J = 5.46 Hz, 2H, CH2–CH2), 3.40 (m, 2H, CH2–CH2), 3.26 (t, J = 4.4 Hz, 1H), 2.13–2.06 (m, 1H), 1.92–1.86 (m, 1H), 1.55–1.43 (m, 1H), 1.88 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.81 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 202.76, 166.45, 162.42, 154.29, 135.72, 125.94, 62.97, 58.27, 47.84, 44.24, 39.61, 29.61, 23.28, 20.34, 17.92, 16.94, 8.80.
CH2), 4.16 (t, J = 5.46 Hz, 2H, CH2–CH2), 3.40 (m, 2H, CH2–CH2), 3.26 (t, J = 4.4 Hz, 1H), 2.13–2.06 (m, 1H), 1.92–1.86 (m, 1H), 1.55–1.43 (m, 1H), 1.88 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.81 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 202.76, 166.45, 162.42, 154.29, 135.72, 125.94, 62.97, 58.27, 47.84, 44.24, 39.61, 29.61, 23.28, 20.34, 17.92, 16.94, 8.80.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1637 (C
O stretching), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1497 (C–H asym stretching), 1301 (C–O–C asym stretching), 1179 (C–O–C sym stretching), 937 (N–O stretching), 716 (C–S stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.06 (d, J = 5.00 Hz, 1H), 7.83 (d, J = 5.04 Hz, 1H), 7.76 (t, J = 5.64 Hz, 1H, NH), 7.65 (d, J = 3.20 Hz, 1H), 7.49 (d, J = 3.04 Hz, 1H), 7.27 (t, J = 4.46 Hz, 1H), 7.22 (t, J = 4.38 Hz, 1H), 6.08 (s, 1H, C
C stretching), 1497 (C–H asym stretching), 1301 (C–O–C asym stretching), 1179 (C–O–C sym stretching), 937 (N–O stretching), 716 (C–S stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.06 (d, J = 5.00 Hz, 1H), 7.83 (d, J = 5.04 Hz, 1H), 7.76 (t, J = 5.64 Hz, 1H, NH), 7.65 (d, J = 3.20 Hz, 1H), 7.49 (d, J = 3.04 Hz, 1H), 7.27 (t, J = 4.46 Hz, 1H), 7.22 (t, J = 4.38 Hz, 1H), 6.08 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.69 (s, 1H, C
CH2), 5.69 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.20 (t, J = 5.46 Hz, 2H, CH2–CH2), 3.45 (m, 2H, CH2–CH2), 1.89 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 166.49, 154.01, 149.51, 135.75, 134.25, 132.96, 131.21, 129.71, 127.70, 126.94, 125.94, 62.89, 39.67, 17.95.
CH2), 4.20 (t, J = 5.46 Hz, 2H, CH2–CH2), 3.45 (m, 2H, CH2–CH2), 1.89 (s, 3H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 166.49, 154.01, 149.51, 135.75, 134.25, 132.96, 131.21, 129.71, 127.70, 126.94, 125.94, 62.89, 39.67, 17.95.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, CH stretching), 2949 (C–H asym stretching), 1753, 1696 (C
CH, CH stretching), 2949 (C–H asym stretching), 1753, 1696 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1632 (C
O stretching), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1519 (C–H asym stretching), 1324, 1222 (C–O–C asym stretching), 1177 (C–O–C sym stretching), 954 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.14 (t, J = 5.68 Hz, 2H, NH), 7.90 (d, J = 0.88 Hz, 2H), 7.85 (d, J = 3.56 Hz, 2H), 6.86 (d, J = 1.92 Hz, 2H), 6.08 (s, 2H, C
C stretching), 1519 (C–H asym stretching), 1324, 1222 (C–O–C asym stretching), 1177 (C–O–C sym stretching), 954 (N–O stretching). 1H NMR (400 MHz, DMSO-d6): δ = 8.14 (t, J = 5.68 Hz, 2H, NH), 7.90 (d, J = 0.88 Hz, 2H), 7.85 (d, J = 3.56 Hz, 2H), 6.86 (d, J = 1.92 Hz, 2H), 6.08 (s, 2H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.69 (s, 2H, C
CH2), 5.69 (s, 2H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.20 (t, J = 5.34 Hz, 4H, CH2–CH2), 3.45 (m, 4H, CH2–CH2), 1.89 (s, 6H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 166.50, 153.64, 146.29, 143.05, 142.74, 135.72, 126.00, 121.66, 113.20, 62.94, 39.59, 17.94.
CH2), 4.20 (t, J = 5.34 Hz, 4H, CH2–CH2), 3.45 (m, 4H, CH2–CH2), 1.89 (s, 6H, CH3). 13C NMR (400 MHz, DMSO-d6): δ = 166.50, 153.64, 146.29, 143.05, 142.74, 135.72, 126.00, 121.66, 113.20, 62.94, 39.59, 17.94.2.3 Synthesis of photobase generating copolymer and preparation of fluorescence patterns
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 10076,
n = 10076, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w/
w/![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 1.34, Tg = 210 °C.
n = 1.34, Tg = 210 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF–PMA mixture. The solution was spin-coated onto glasses for 7 s at 1500 rpm followed by 10 s at 2000 rpm to form copolymer films. The films were dried at 40 °C for 5 h under reduced pressure. The thickness of the obtained films ranged from 0.9 to 1.2 μm. The films on the glass were covered with dot/line array photomasks and irradiated with 254 nm UV light (1.05 J cm−2). The irradiated films on the glass were dipped in a 3.0 mM fluorescamine solution (8.62 mg in 10 mL of a 4
1 (v/v) THF–PMA mixture. The solution was spin-coated onto glasses for 7 s at 1500 rpm followed by 10 s at 2000 rpm to form copolymer films. The films were dried at 40 °C for 5 h under reduced pressure. The thickness of the obtained films ranged from 0.9 to 1.2 μm. The films on the glass were covered with dot/line array photomasks and irradiated with 254 nm UV light (1.05 J cm−2). The irradiated films on the glass were dipped in a 3.0 mM fluorescamine solution (8.62 mg in 10 mL of a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) cyclohexane–acetone mixture) for 1 min and subsequently dried at 50 °C for 1 h. The fluorescence images were observed using confocal microscopy.
1 (v/v) cyclohexane–acetone mixture) for 1 min and subsequently dried at 50 °C for 1 h. The fluorescence images were observed using confocal microscopy.2.4 Instruments and measurements
Attenuated total refraction Fourier-transform infrared spectroscopy (ATR FT-IR) was obtained using a Perkin Elmer RX1 spectrometer produced by Thermo Corporation at frequencies from 500 to 4000 cm−1. The fractional transmittance changes (ΔT) in FT-IR spectra of the four monomers were determined from the difference in transmittance at certain wavenumbers after irradiation for a given period. The ΔT was determined from the following equation:
 | (1) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O represents the transmittance peak of SMC at 1737 cm−1, ACMC at 1719 cm−1, TKMC at 1705 cm−1 and DFMC at 1696 cm−1. Proton nuclear magnetic resonance (1H and 13C NMR, 400 MHz) spectra were recorded on a Bruker AV400 spectrometer using DMSO-d6 as solvent. Ultraviolet-visible (UV-Vis) absorption spectra were recorded on a Hitachi U-3010 spectrophotometer. The absorbance of SMC, ACMC, TKMC and DFMC solutions in THF at λmax were adjusted to 0.90–1.10 before irradiation with 254 nm UV light. The relative fluorescence intensity of the irradiated solutions after treatment with fluorescamine was measured on a Hitachi F-4500 fluorescence spectrophotometer. Gel permeation chromatography (GPC) analysis was carried out on a Waters 515-2410 system using polystyrene standard as the molecular weight reference and THF as the eluent. Differential scanning calorimetry (DSC) measurements were performed on a TA Q20 system at a heating rate of 20 °C min−1 under a nitrogen atmosphere. The thickness of the polymer films was measured using an α-step surface profiler (Tencor Instruments, Model AS-500). A Philips lamp (PL-L18W), equipped with a 254 nm fluorescent lamp, was placed in a horizontal position and used for the irradiation process. The light intensity measured by a radiometer was 3.50 mW cm−2. The fluorescence images were observed using an Olympus-IX81 fluorescence microscope with an exposure time of 1.2 s.
O represents the transmittance peak of SMC at 1737 cm−1, ACMC at 1719 cm−1, TKMC at 1705 cm−1 and DFMC at 1696 cm−1. Proton nuclear magnetic resonance (1H and 13C NMR, 400 MHz) spectra were recorded on a Bruker AV400 spectrometer using DMSO-d6 as solvent. Ultraviolet-visible (UV-Vis) absorption spectra were recorded on a Hitachi U-3010 spectrophotometer. The absorbance of SMC, ACMC, TKMC and DFMC solutions in THF at λmax were adjusted to 0.90–1.10 before irradiation with 254 nm UV light. The relative fluorescence intensity of the irradiated solutions after treatment with fluorescamine was measured on a Hitachi F-4500 fluorescence spectrophotometer. Gel permeation chromatography (GPC) analysis was carried out on a Waters 515-2410 system using polystyrene standard as the molecular weight reference and THF as the eluent. Differential scanning calorimetry (DSC) measurements were performed on a TA Q20 system at a heating rate of 20 °C min−1 under a nitrogen atmosphere. The thickness of the polymer films was measured using an α-step surface profiler (Tencor Instruments, Model AS-500). A Philips lamp (PL-L18W), equipped with a 254 nm fluorescent lamp, was placed in a horizontal position and used for the irradiation process. The light intensity measured by a radiometer was 3.50 mW cm−2. The fluorescence images were observed using an Olympus-IX81 fluorescence microscope with an exposure time of 1.2 s.
3. Results and discussion
3.1 Photobase generating properties of the four monomers
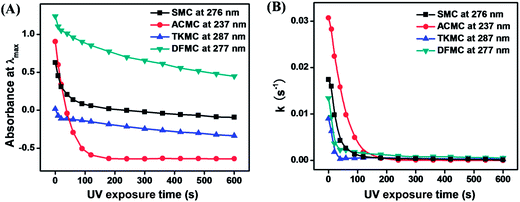
Scheme 1 shows the reaction process for the synthesis of the four monomers used here along with their chemical structures. The photobase generating properties of the monomers have been studied by observing changes in their UV-Vis absorption spectra, FT-IR spectra and fluorescence spectra after treatment with fluorescamine, and changes in pH values.Fig. 1 shows changes in the UV-Vis absorption spectra of the four monomers in THF upon irradiation with 254 nm UV light. The absorption band at 250–300 nm decreases upon irradiation owing to the photodecomposition of the carbamate group.21–24 Correspondingly, the band at 300–350 nm increases upon UV irradiation due to the formation of photoproducts. The photodecomposition rate of the carbamate group for the four monomers can be suggested by the rate constant (k), which was determined from the following equation:
 | (2) |
 | ||
| Fig. 1 Changes in the UV-Vis absorption spectra of (A) SMC, (B) ACMC, (C) TKMC and (D) DFMC in THF upon irradiation with 254 nm UV light for 0–600 s. | ||
To confirm the photolysis products, FT-IR was performed. The FT-IR spectra of the solvent THF and fluorescamine solution in THF (lines 1 and 2 in Fig. 3(A–D)) were introduced as references. Lines 3 and 4 in Fig. 3(A–D) show changes of FT-IR spectra for 5 mM solutions of the monomers in THF before and after irradiation with 254 nm UV light. After irradiation for 30 min, the peak at 3307 cm−1 increased for SMC (line 4 in Fig. 3A) compared with that before irradiation (line 3 in Fig. 3A). This demonstrates the formation of amino groups after UV irradiation. The decrease of the peak at 1781 cm−1 results from the photodecomposition of carbonyl units. After treatment with fluorescamine (line 5 in Fig. 3A), the appearance of the peak at 1565 cm−1 indicates the formation of fluorescamine–amine adducts. Similarly, after UV irradiation the peak at 3342 cm−1 increased, whereas the peak at 1761 cm−1 decreased for ACMC (line 4 in Fig. 3B). However, it is worth mentioning that a new peak at 2237 cm−1 was observed after UV irradiation for ACMC (line 4 in Fig. 3B), which suggests the formation of isocyanato groups. This may result from the thermal instability of the camphorquinone carbamate group.26 The FT-IR changes for TKMC (Fig. 3C) and DFMC (Fig. 3D) are similar to those of SMC. The FT-IR spectra demonstrate the photodecomposition process and confirm the photoproducts, which suggest stable photochemical properties of the four monomers.
Fig. 4A shows UV-Vis absorption spectral changes in the fluorescamine solution in THF before and after reacting with amino groups arising from the irradiated solution of the four monomers in THF. The UV-Vis absorption spectrum of the fluorescamine solution after reacting with amino groups presented a new absorption band around 400 nm (inset of Fig. 4A). Its appearance demonstrates the formation of fluorescamine–amine adducts.21 In order to investigate the properties of fluorescamine–amine adducts, fluorescence spectra were obtained. Fig. 4B shows the relative fluorescence intensities of fluorescamine treated monomer solutions in THF at 476 nm as a function of UV exposure time. The fluorescence intensity of ACMC and DFMC increases as the irradiation time increases and reaches a peak at 6 min before gradually decreasing, whereas that of SMC reaches a peak at 10 min and keeps stable even after irradiation for 30 min. The obvious and quick decrease for ACMC is possibly caused by the formation of isocyanato groups during the irradiation.23 Isocyanato groups can react with amino groups, which leads to a decrease of amino groups in the solution before treatment with fluorescamine. However, the relatively slow decrease for DFMC may be due to the self-quenching reaction of the fluorescamine–amine adducts at high concentrations of the amino groups23 since there are no isocyanato groups observed after irradiation for DFMC.
To study the effect of the isocyanato group on the fluorescence intensity of the monomers, we compared the fractional transmittance changes (ΔT) among the four monomers at different wavenumbers in Fig. 5 (A–C). As is known, the reaction process can be monitored by the decrease of the carbonyl peaks and the increase of the amino peaks in the FT-IR spectra. The ΔT of carbonyl peaks (Fig. 5A) indicates that ACMC has higher reaction rate than SMC, which is in agreement with the order of k values and the fluorescence spectral data for the four monomers. However, the ΔT of amino peaks (Fig. 5B) of ACMC is lower than that of SMC. Meanwhile, the isocyanato peak (Fig. 5C) increases at 6 min for ACMC, whereas no isocyanato peak is observed even after irradiation for 30 min for SMC. Therefore, it is reasonable to conclude that the isocyanato groups consume the amino groups during the irradiation, which results in the decrease of amino groups and the decrease of the fluorescence intensity after treatment with fluorescamine.23
 | ||
| Fig. 5 Fractional transmittance changes of the monomer solutions for carbonyl (A), amino (B) and isocyanato (C) peaks. (D) Changes in the pH values of 1 mM THF solutions of the monomers. | ||
Self-quenching experiments of fluorescamine–amine adducts using alkylamines show that the maximum fluorescence intensity is observed at an alkylamine concentration of 0.1 mM, in which solution the pH value is about 8.5–9.0.23 So the relative concentrations of amines formed from the photobase generating monomers upon UV irradiation have been further studied by observing changes in their pH values. Fig. 5D shows the pH changes of a 1 mM THF solution for the four monomers. The pH values of their solutions increase from ca. 6.2–7.1 to ca. 7.9–8.8 after UV irradiation, suggesting the generation of amines. The pH values for DFMC are higher than those of SMC, ACMC and TKMC after UV irradiation. This difference originates from the two carbamate groups in DFMC. It is worth mentioning that only the pH value of DFMC solution reaches 8.7, which is in the self-quenching pH value range of the alkylamine. Therefore, the explanations regarding the decreasing trend for the relative fluorescence intensity of ACMC and DFMC are reasonable.
3.2 Fluorescence patterning based on copolymer from photobase generating monomer
In order to apply the photobase generating properties of the monomers to a fluorescence image recording material, a copolymer containing both SMC and MMA with the mole ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 has been prepared. Here SMC was chosen due to its relatively quick rate of photodecomposition and most stable photobase generating property among all monomers as discussed above, and MMA was introduced to decrease the steric effect23,24 of the succinimido groups in SMC. The copolymer shows good solubility in THF, so a uniform thin transparent film was obtained from the copolymer solution in THF.
7 has been prepared. Here SMC was chosen due to its relatively quick rate of photodecomposition and most stable photobase generating property among all monomers as discussed above, and MMA was introduced to decrease the steric effect23,24 of the succinimido groups in SMC. The copolymer shows good solubility in THF, so a uniform thin transparent film was obtained from the copolymer solution in THF.
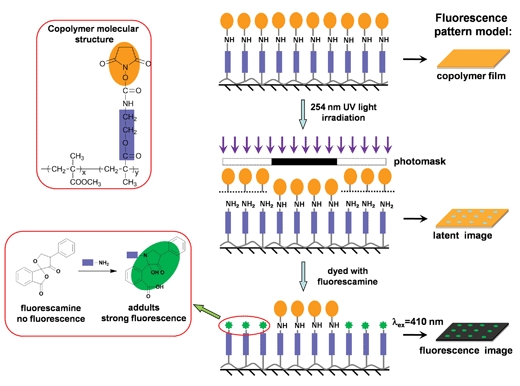
Fig. 6 shows a schematic depiction of the process for the formation of fluorescence patterns. A thin copolymer film formed on a glass substrate via spin-coating is covered with a dot or line array photomask and irradiated with 254 nm UV light. A latent image is formed in the irradiated area through the formation of amino groups, which arise from the photodecomposition of the SMC groups in the copolymer. The consecutive treatment of this latent image with fluorescamine leads to the formation of a dyed image. The dyed image can be observed by confocal microscopy. Different colored fluorescence patterns are observed via the selection of different observation wavelengths.
The fluorescence spectrum of the copolymer film (Fig. 7A), upon irradiation with 254 nm UV light followed by reaction with fluorescamine, is characterized by a fluorescence band in the range of 420–650 nm with a fluorescence maximum at 476 nm. This result indicates that amino groups are produced upon irradiation, since fluorescamine itself is not fluorescent and only becomes fluorescent after reaction with the amino groups. According to the emission filter used in the confocal microscopy, we divided the emission spectrum of the fluorescamine–amine adducts into three parts: 420–470, 470–550 and 550–650 nm,24 which correspond to blue, green and red observation colors, respectively. Fluorescence patterns obtained with the copolymer films on glasses demonstrate blue, green and red colors in certain observation wavelength ranges as shown in Fig. 7(B–G). The fluorescence patterns based on our photobase generating system appeared as visible fluorescence with high contrast and resolution under a smaller exposure dose compared with those in literature.21–24 This system may be useful in the preparation of fluorescence images in polymer films for photonic and optical applications.
4. Conclusions
To sum up, four novel carbamate functional photobase generating monomers, SMC, ACMC, TKMC and DFMC, have been synthesized successfully for fluorescence imaging applications. The photochemical characteristics of the synthesized monomers can be adjusted by altering the photosensitive groups. The experimental results indicated by the changes in UV-Vis spectra, FT-IR spectra, fluorescence intensities, and pH values suggest that SMC has the best properties for producing amines upon irradiation with 254 nm UV light among the four monomers. Furthermore, a copolymer containing SMC and MMA is used as a fluorescence imaging material. The fluorescence images formed on the copolymer film show different visible fluorescence colors, such as blue, green or red, depending upon the observation wavelength. This kind of material may be useful in the preparation of fluorescence images in polymer films for photonic and optical applications.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant no. 51173013 and 51373013).Notes and references
- E. Y. Park, J. W. Kim, D. J. Ahn and J. M. Kim, Macromol. Rapid Commun., 2007, 28, 171 CrossRef CAS.
- P. F. Tian, P. E. Burrows and S. R. Forrest, Appl. Phys. Lett., 1997, 71, 3197 CrossRef CAS PubMed.
- (a) G. Pistolis, S. Boyatzis, M. Chatzichristidi and P. Argitis, Chem. Mater., 2002, 14, 790 CrossRef CAS; (b) K. D. Ahn, K. W. Yoo, J. H. Soh and J. H. Kang, React. Polym., 2009, 69, 111 CrossRef CAS PubMed; (c) J. Bharathan and Y. Yang, Appl. Phys. Lett., 1998, 72, 2660 CrossRef CAS PubMed; (d) S. J. Lim, J. Seo and S. Y. Park, J. Am. Chem. Soc., 2006, 128, 14542 CrossRef CAS PubMed.
- (a) T. Renkecz, G. Mistlberger, M. Pawlak, V. Horváth and E. Bakker, ACS Appl. Mater. Interfaces, 2013, 5, 8537 CrossRef CAS PubMed; (b) D. S. Achilleos, T. A. Hatton and M. Vamvakaki, J. Am. Chem. Soc., 2012, 134, 5726 CrossRef CAS PubMed; (c) T. F. Masahiro Irie, N. T. Takatoshi Sasaki and T. Kawai, Nature, 2002, 420, 759 CrossRef PubMed.
- (a) S. Ryu, I. Yoo, S. Song, B. Yoon and J. M. Kim, J. Am. Chem. Soc., 2009, 131, 3800 CrossRef CAS PubMed; (b) Z. Orynbayeva, S. Kolusheva, E. Livneh, A. Lichtenshtein, I. Nathan and R. Jelinek, Angew. Chem., Int. Ed., 2005, 44, 1092 CrossRef CAS PubMed; (c) K. Sun, X. Liu, Y. Wang and Z. Wu, RSC Adv., 2013, 3, 14543 RSC; (d) E. Nakata, Y. Yukimachi, Y. Nazumi, M. Uwate, H. Maseda, Y. Uto, T. Hashimoto, Y. Okamoto, H. Hori and T. Morii, RSC Adv., 2014, 4, 348 RSC; (e) Q. C. Xu, X. F. Wang, G. W. Xing and Y. Zhang, RSC Adv., 2013, 3, 15834 RSC; (f) Q. C. Xu, X. H. Zhu, C. Jin, G. W. Xing and Y. Zhang, RSC Adv., 2014, 4, 3591 RSC; (g) Y. Du, Y. Zhang, K. Huang, S. Wang, L. Yuan and S. Feng, Dalton Trans., 2013, 42, 8041 RSC; (h) N. Na, T. Liu, S. Xu, Y. Zhang, D. He, L. Huang and J. Ouyang, J. Mater. Chem. B, 2013, 1, 787 RSC; (i) J. Jia, Q. C. Xu, R. C. Li, X. Tang, Y. F. He, M. Y. Zhang, Y. Zhang and G. W. Xing, Org. Biomol. Chem., 2012, 10, 6279 RSC; (j) Z. Y. Gu, X. T. Zhang, J. X. Zhang and G. W. Xing, Org. Biomol. Chem., 2013, 11, 5017 RSC.
- (a) L. Huang, J. Su, D. Zhong, H. Wang, R. Liu, L. Yu, Q. Zhu and S. Liu, RSC Adv., 2013, 3, 13286 RSC; (b) F. Gao, J. Lei and H. Ju, RSC Adv., 2013, 3, 13163 RSC; (c) Y. Ooyama, K. Uenaka, A. Matsugasako, Y. Harima and J. Ohshita, RSC Adv., 2013, 3, 23255 RSC; (d) R. Kang, X. Shao, F. Peng, Y. Zhang, G. T. Sun, W. Zhao and X. D. Jiang, RSC Adv., 2013, 3, 21033 RSC; (e) S. Wuttke, C. Dietl, F. M. Hinterholzinger, H. Hintz, H. Langhals and T. Bein, Chem. Commun., 2014, 50, 3599 RSC; (f) G. Baldacchino and A. Balcerzyk, Analyst, 2014, 139, 1707 RSC.
- (a) J. Lee, C. W. Lee and J. M. Kim, Macromol. Rapid Commun., 2010, 31, 1010 CrossRef CAS PubMed; (b) J. Kim, J. Cho, J. Lee, K. Park and J. M. Kim, Macromol. Rapid Commun., 2011, 32, 870 CrossRef CAS PubMed; (c) M. L. J. C. Scaiano and M. G. Ivan, Macromolecules, 2003, 38, 6692 CrossRef; (d) A. Miyawaki, Microscopy, 2013, 62, 63 CrossRef CAS PubMed.
- (a) P. G. Atsushi Aoki and R. M. Crooks, Langmuir, 1999, 15 Search PubMed; (b) W. S. Dillmore, M. N. Yousaf and M. Mrksich, Langmuir, 2004, 20, 7223 CrossRef CAS PubMed; (c) S. Natarajan and S. H. Kim, Langmuir, 2005, 21, 7052 CrossRef CAS PubMed; (d) K. L. Christman, M. V. Requa, V. D. Enriquez-Rios, S. C. Ward, K. A. Bradley, K. L. Turner and H. D. Maynard, Langmuir, 2006, 22, 7444 CrossRef CAS PubMed; (e) M. S. Hahn, J. S. Miller and J. L. West, Adv. Mater., 2006, 18, 2679 CrossRef CAS.
- B. A. Langowski and K. E. Uhrich, Langmuir, 2005, 21, 10509 CrossRef CAS PubMed.
- (a) H. Y. Shim, S. H. Lee, D. J. Ahn, K. D. Ahn and J. M. Kim, Mater. Sci. Eng., C, 2004, 24, 157 CrossRef PubMed; (b) C. H. Zhang, A. M. Vekselman and G. D. Darling, Chem. Mater., 1995, 7, 850 CrossRef CAS; (c) Z. N. Bao, J. A. Rogers and H. E. Katz, J. Mater. Chem., 1999, 9, 1895 RSC.
- (a) W. Wang, C. Du, L. Li, H. Wang, C. Wang, Y. Wang, H. Fuchs and L. Chi, Adv. Mater., 2013, 25, 2018 CrossRef CAS PubMed; (b) W. Hu, N. Lu, S. Shi, Y. Wang, Y. Wang, Z. Cui, X. Huang, Y. Liu, M. Xu and L. Chi, Langmuir, 2009, 25, 4352 CrossRef CAS PubMed; (c) L. Gao, N. Lu, J. Hao, W. Hu, G. Shi, Y. Wang and L. Chi, Langmuir, 2009, 25, 3894 CrossRef CAS PubMed.
- (a) W. Wang, C. Du, H. Bi, Y. Sun, Y. Wang, C. Mauser, E. D. Como, H. Fuchs and L. Chi, Adv. Mater., 2010, 22, 2764 CrossRef CAS PubMed; (b) W. Wang, C. Du, C. Wang, M. Hirtz, L. Li, J. Hao, Q. Wu, R. Lu, N. Lu, Y. Wang, H. Fuchs and L. Chi, Small, 2011, 7, 1403 CrossRef CAS PubMed; (c) W. Hu, N. Lu, H. Zhang, Y. Wang, N. Kehagias, V. Reboud, C. M. Sotomayor Torres, J. Hao, W. Li, H. Fuchs and L. Chi, Adv. Mater., 2007, 19, 2119 CrossRef CAS.
- S. Y. Cho, Y. K. Song, J. G. Kim, S. Y. Oh and C. M. Chung, Tetrahedron Lett., 2009, 50, 4769 CrossRef CAS PubMed.
- (a) J. M. Kim, Y. B. Lee, S. K. Chae and D. J. Ahn, Adv. Funct. Mater., 2006, 16, 2103 CrossRef CAS; (b) D. J. Ahn and J. M. Kim, Acc. Chem. Res., 2008, 41, 805 CrossRef CAS PubMed.
- (a) G. Kwak, M. Fujiki, T. Sakaguchi and T. Masuda, Macromolecules, 2005, 39, 319 CrossRef; (b) H. Park, H. Jeong, W. E. Lee, K. B. Yoon, C. J. Oh and G. Kwak, Macromol. Rapid Commun., 2011, 32, 360 CrossRef CAS PubMed; (c) H. Park, D. C. Han, D. H. Han, S. J. Kim, W. E. Lee and G. Kwak, Macromolecules, 2011, 44, 9351 CrossRef CAS.
- (a) J. M. Kim, T. E. Chang, J. H. Kang, D. K. Han and K. D. Ahn, Adv. Mater., 1999, 11, 1499 CrossRef CAS; (b) J. H. Yoo, S. Y. Kim, I. Cho, J. M. Kim, K. D. Ahn and J. H. Lee, Polymer, 2004, 45, 5391 CrossRef CAS PubMed; (c) S. Park, S. Kim, J. Seo and S. Y. Park, Macromolecules, 2005, 38, 4557 CrossRef CAS; (d) C. W. Lee, Z. Yuan, K. D. Ahn and S. H. Lee, Chem. Mater., 2002, 14, 4572 CrossRef CAS.
- (a) C. Coenjarts, O. García, L. Llauger, J. Palfreyman, A. L. Vinette and J. C. Scaiano, J. Am. Chem. Soc., 2002, 125, 620 CrossRef PubMed; (b) J. Mravljak, T. Ojsteršek, S. Pajk and M. Sollner Dolenc, Tetrahedron Lett., 2013, 54, 5236 CrossRef CAS PubMed.
- (a) M. Shirai and M. Tsunooka, Prog. Polym. Sci., 1996, 21, 1 CrossRef CAS; (b) K. Suyama and M. Shirai, Prog. Polym. Sci., 2009, 34, 194 CrossRef CAS PubMed.
- S. K. Lee, B. J. Jung, T. Ahn, I. Song and H. K. Shim, Macromolecules, 2003, 36, 9252 CrossRef CAS.
- T. Griesser, S. V. Radl, T. Koepplmayr, A. Wolfberger, M. Edler, A. Pavitschitz, M. Kratzer, C. Teichert, T. Rath, G. Trimmel, G. Schwabegger, C. Simbrunner, H. Sitter and W. Kernab, J. Mater. Chem., 2012, 22, 2922 RSC.
- (a) K. H. Chae, G. J. Sun, J. K. Kang and T. H. Kim, J. Appl. Polym. Sci., 2002, 86, 1172 CrossRef CAS; (b) K. H. Chae and J. Park, Macromol. Res., 2004, 12, 352 CrossRef CAS; (c) K. H. Chae, H. I. Cho, Y. H. Kim and U. C. Yang, Eur. Polym. J., 2012, 48, 1186 CrossRef CAS PubMed.
- W. S. Choi, Y. Y. Noh and K. H. Chae, Adv. Mater., 2005, 17, 833 CrossRef CAS.
- K. H. Chae and Y. H. Kim, Adv. Funct. Mater., 2007, 17, 3470 CrossRef CAS.
- K. H. Chae and S. J. Baek, Macromol. Chem. Phys., 2012, 213, 1190 CrossRef CAS.
- (a) S. Abbate, L. F. Burgi, F. Gangemi, R. Gangemi, F. Lebon, G. Longhi, V. M. Pultz and D. A. Lightner, J. Phys. Chem. A, 2009, 113, 11390 CrossRef CAS PubMed; (b) M. F. N. N. Carvalho, A. M. Galvão, J. Kredatusová, J. Merna, P. F. Pinheiro and M. M. a. Salema, Inorg. Chim. Acta, 2012, 383, 244 CrossRef CAS PubMed.
- R. Boobalan, C. Chen and G. Lee, Org. Biomol. Chem., 2012, 10, 1625 CAS.
| This journal is © The Royal Society of Chemistry 2014 |