Amelioration of sodium arsenite induced toxicity by diallyl disulfide, a bioactive component of garlic: the involvement of antioxidants and the chelate effect†
Abstract
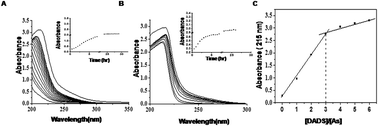
Chronic exposure to high concentrations of arsenic in drinking water poses severe health problems. Despite arsenic being a severe health hazard, a safe and effective remedy against arsenic poisoning remains elusive. Previously, studies from our group showed that aqueous garlic extract could be a potential protective substance against arsenic toxicity. In the present study, we aimed to identify the bioactive component of garlic that participates in the remediation of arsenic toxicity. To this end we studied the interaction between diallyl disulphide (DADS), a stable antioxidant present in garlic, with NaAsO2 by UV spectrophotometry. The UV spectral data revealed the chemical interactions between DADS and As(III) in a 3 : 1 molar ratio. Furthermore, we investigated the potency of DADS in combating arsenic toxicity in human hepatocellular carcinoma cell line (HepG2) by MTT assay. The results showed that 50 μM DADS along with 10 μM NaAsO2 could effectively attenuate the arsenite-induced cytotoxicity, production of reactive oxygen species (ROS), lipid peroxidation and DNA damage. Application of DADS along with NaAsO2 in HepG2 cells resulted in the modulation of arsenite-induced activities of antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) to near normal levels. Finally, we attempted to trace the chemical interaction between As(III) and DADS in the HepG2 cellular environment. High-performance liquid chromatography (HPLC) analysis of the NaAsO2 and DADS treated cell lysate revealed similar interactions between NaAsO2 and DADS to those observed in vitro.

 Please wait while we load your content...
Please wait while we load your content...