Defect controlled water splitting characteristics of gold nanoparticle functionalized ZnO nanowire films
Abstract
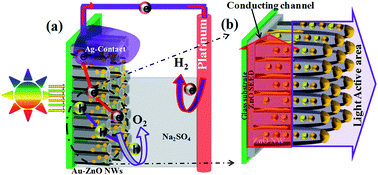
Functionalization of ZnO nanowires by size controlled gold nanoparticles is demonstrated through sputtering of a gold layer followed by annealing at different temperature. Rationally designed sputtered Au nanoparticle–ZnO nanowire (AuNP–ZnO) exhibits high photocatalytic activity for water splitting. The tailored structure shows an enhanced photocurrent of 300 μA cm−2 under visible light illumination. Additionally, the efficiency of 0.13% at a low bias of 0.45 V vs. Ag–AgCl indicates an efficient charge separation and collection without a conducting ITO/FTO base. The AuNPs tune the visible light absorption and utilize the SPR of the Au nanoparticle to boost the hot electron injection from Au nanoparticles to the ZnO conduction band. Moreover, AuNPs also assist in the suppression of surface defects of ZnO NWs (or hole traps) significantly and enhance the water splitting performance of the material under visible light.

 Please wait while we load your content...
Please wait while we load your content...