Rhodomyrtals A–D, four unusual phloroglucinol-sesquiterpene adducts from Rhodomyrtus psidioides†
Qingyao Shou*a,
Joshua E. Smitha,
Htwe Monb,
Zlatko Brkljačac,
Ana-Sunčana Smithcd,
David M. Smithcd,
Hans J. Griesserb and
Hans Wohlmutha
aSouthern Cross Plant Science, Southern Cross University, PO Box 157, Lismore NSW 2480, Australia. E-mail: sanqi_0617@126.com
bIan Wark Research Institute, University of South Australia, Mawson Lakes SA 5095, Australia
cInstitute for Theoretical Physics, Friedrich Alexander University Erlangen-Nürnberg, Nägelsbachstrasse 49b, Erlangen, 91052, Germany
dRuđer Bošković Institute, Bijenička 54, 10000 Zagreb, Croatia
First published on 28th February 2014
Abstract
Four novel compounds, rhodomyrtals A–D (1–4), with two unprecendented carbon frameworks of phloroglucinol coupled eudesmane, together with the known compound eucalyptin A (5) have been isolated from the leaves of the Australian plant Rhodomyrtus psidioides. The structures of compounds 1–4 were elucidated by spectroscopic analysis and ECD calculations. Some of the compounds showed good antibacterial activity against selected Gram-positive strains.
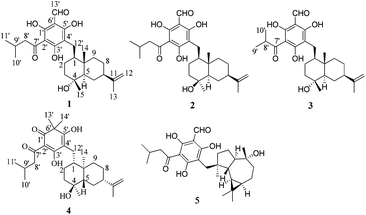
The genus Rhodomyrtus (Myrtaceae) consists of 17 species, which are native to Asia, Malesia, Melanesia, and Australia.1,2 During the past few years, a number of unusual phloroglucinol derivatives such as rhodomyrtone,3 rhodomyrtosones,4 and tomentosones,5 have been isolated from Rhodomyrtus tomentosa. Among them, rhodomyrtone displayed potent anti-bacterial activity, especially against methicillin-resistant Staphylococcus aureus.6 In our efforts to discover natural products with potential application in wound healing, we have examined the Australian endemic species Rhodomyrtus psidioides (G. Don) Benth. This species, commonly called native guava, is a shrub or small rainforest tree, which grows up to 12 m high, native to eastern Australia.7 Chemical investigations of the leaves of this plant led to the isolation of four novel compounds (1–4) (Fig. 1) that possess two new phloroglucinol-coupled eudesmane skeletons, as well as the known compound eucalyptin A (5). Herein, details of the isolation, structural elucidation, and antibacterial activity of the rhodomyrtals A–D (1–4) are described.
Compound 1 was obtained as a pale yellow oil. It showed a [M − H]− peak at m/z 471.2759 (calcd 471.2747) by high-resolution electrospray-ionization mass spectrometry (HRESIMS), corresponding to a molecular formula of C28H40O6 with 9 degrees of unsaturation. The IR spectrum of 1 indicated the presence of carbonyl at 1616 cm−1. From an inspection of 1D-NMR data (Table S1†) and the HSQC spectrum, 1 was found to possess a 3-methybutanoyl side chain [δH 3.18 (2H, m), 2.40 (1H, m), 1.03 (6H, d, J = 6.6 Hz); δC 206.9, 53.4, 25.7, 23.3, 23.3], a phloroglucinol unit [δC 172.5, 108.9, 167.1, 106.7, 169.6, 105.5] bearing an aldehyde [δH 10.54 (1H, s), δC 193.2], one methylene [δH 3.06 (1H, m), 2.71 (1H, m); δC 22.8], three tertiary methyl [δH 0.92 (3H, s), 1.28 (3H, s) and 1.77 (3H, s); δC 15.1, 23.6 and 21.5], a terminal double bond [δH 4.88 and 4.81 (each 1H, s); δC 108.8, 151.5] as well as an oxygenated carbon at δC 71.3. The aforementioned data suggested a phloroglucinol-coupled sesquiterpenoid for compound 1. The above mentioned functionalities accounted for 7 out of the 9 double-bond equivalents, which indicated the presence of two rings in compound 1.
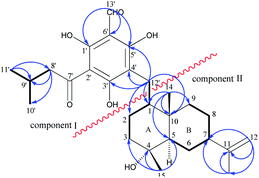
The key HMBC correlations (Fig. 2) of H-13′ with C-1′ (δC 169.6), C-6′ (δC 106.7) and C-5′ (δC 167.1) as well as of H-12′ with C-5′ (δC 167.1), C-3′ (δC 172.5) and C-4′ (δC 108.9) located the aldehyde and the C-12′ methylene at positions 6′ and 4′, respectively, and also allowed the assignment of three hydroxyls at C-1′, C-3′ and C-5′. Consequently, the 3-methylbutanoyl substituent could only be placed at C-2′. Thus, a grandinol moiety (Fig. 2, component I) was established based on this analysis above, which was also supported by comparison with the NMR data of grandinol.8
In the 1H–1H COSY spectrum, homo-nuclear coupling correlations led to the establishment of two structural fragments (C-7′ to C-3 and C-5 to C-9) as drawn with bold bonds in Fig. 2. The connections of the two structural fragments, quaternary carbons, and the other functional groups were mainly achieved by the HMBC experiment. The HMBC correlations (Fig. 2) of H3-14 with C-1, C-5, C-9 and C-10 suggested attachment of C-1, C-5, and C-9 to the quaternary carbon C-10 to furnish a six-membered ring B and also indicated the attachment of Me-14 to C-10. The HMBC correlations from H3-15 to C-3, C-4, and C-5 incorporated an oxygenated quaternary carbon between C-3 and C-5 to establish the other six-membered ring A and also allowed assignment of Me-15 to C-4. An isopropenyl group was attached to C-7 by the HMBC correlations of H2-12 with C-7, C-11, and C-13, and of H3-13 with C-7 and C-11. Thus, a sesquiterpenoid moiety of 11-eudesmene-4-ol (Fig. 2, component II) in compound 1 was fully established based on the above spectral analysis. Component I and II were linked to each other via the C-1-C-12′ bond as judged by the HMBC correlations of H2-12′ with C-1, C-2 and C-10.
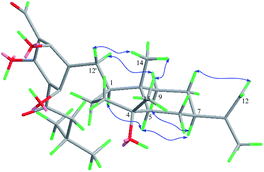
The relative stereochemistry of 1 was established on the basis of a phase-sensitive NOESY experiment (Fig. 3). The correlations of CH3-14/H-12′ and CH3-14/CH3-15 indicated that CH2-12′, CH3-14 and CH3-15 were in a β-orientation, and subsequently the NOESY cross peaks of H-1/H-5 and H-5/H-7 revealed that H-1, H-5 and H-7 should be assigned to be α-oriented. The absolute configuration of 1 was deduced by comparison of the experimental and the calculated ECD spectra. The theoretical prediction of the ECD spectra of (1S,4R,5R,7R,10S)-1 and its enantiomer were obtained on the basis of a method described in ref. 9, using the TD-B3LYP/6-31G+(d) level of theory (see ESI† for details). The calculated spectra of (1S,4R,5R,7R,10S)-1 compares well with the experimental ECD (measured in methanol) in the region of 200–400 nm (Fig. 4a). The absolute configuration of compound 1 was thus assigned as depicted.
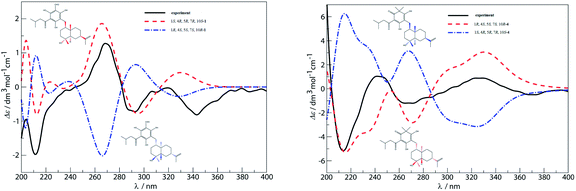 | ||
| Fig. 4 Experimental ECD spectra of compounds 1 and 4 (black, (a) and (b) respectively) and the corresponding calculated ECD spectra calculated using TD-B3LYP (red dashed lines, (a) and (b) respectively). See ESI† for details. | ||
Compound 2, a pale yellow oil, had a molecular formula of C28H40O6 as determined by HREIMS at m/z 471.2756 [M − H]− (calcd 471.2747), indicating that it was an isomer of 1. The IR and UV data of compound 2 were closely related to those of compound 1. The 13C NMR data of 2 also showed high similarity to those of 1, except that the signal for C-15 shifted from δC 23.6 to δC 31.2, and signals for C-2, C-3 and C-5 shifted from δC 26.2, 44.4 and 56.5 to δC 24.3, 42.7 and 53.9 respectively, which indicated a different configuration at the position of C-4. In the NOESY spectrum, the correlations of CH3-15/H-5 and H-5/H-7 supported the inference of α-orientation of CH3-15. Detailed 2D NMR analysis (1H–1H COSY, HMBC, HMQC, and NOESY) confirmed the structure of compound 2. The absolute configuration of compound 2 was established in a manner analogous to that of compound 1.
Compound 3, obtained as a yellow oil, had a molecular formula of C27H38O6 as determined by HRESIMS at m/z 457.2592 [M − H]− (calcd 457.2590), which indicated an analogue of 1 missing a CH2 unit. The 1H and 13C NMR data of 3 (Table S1†) bear a resemblance to those of 1, with the notable difference of the side chain attached to the phloroglucinol unit. The proton signals at δH 4.27 (1H, m) and δH 1.29 (6H, overlap) as well as the carbon signals at δC 211.3, 39.6, 19.8, 19.8 suggested a 2-methylpropionyl moiety for 3 instead of the 3-methybutanoyl side chain in 1. Thus, the planar structure of 3 was determined as depicted and the stereochemistry was established to be the same as that of 1 from the phase-sensitive NOESY spectrum as well as from the CD spectrum.
Compound 4, was obtained as a pale yellow oil. The HRESIMS displayed a [M − H]− peak at m/z 471.3107 (calcd 471.3116), which was consistent with a molecular formula of C29H44O5. A careful comparison of chemical shift values of 4 with those of 1 revealed that 4 contains the same sesquiterpene unit of 11-eudesmene-4-ol. The 1D NMR data (Table S1†) and HSQC spectrum revealed the presence of an unit of 3,5-dihydroxy cyclohexadienone [δC 197.2, 106.4, 189.6, 107.8, 179.2, 49.0] bearing two tertiary methyls [δH 1.66 (3H, s), and 1.70 (3H, s); δC 25.4, 25.3] and a 3-methybutanoyl side chain [δH 3.17 (2H, m), 2.35 (1H, m), 1.02 (6H, d, J = 6.7 Hz); δC 203.2, 49.0, 26.1, 23.2, 23.2]. The HMBC correlations of H3-13′ and H3-14′ with C-1′ (δC 197.2), C-6′ (δC 49.0) and C-5′ (δC 179.2) as well as of H-12′ with C-3′ (δC 189.6), C-4′ (δC 107.8) and C-5′ (δC 179.2) located the two tertiary methyls and the C-12′ methylene at position 6′ and 4′, respectively, and also allowed the assignment of two hydroxyls at C-3′ and C-5′. Thus, a moiety of 3,5-dihydroxy-6,6-dimethyl-2-(3-methyl-1-oxobutyl)-2,4-cyclohexadien-1-one was established based on the analysis above. The linkage of the phloroglucinol and the sesquitepe units was determined through the C-12′ methylene by the HMBC correlations of H2-12′ with C-1, C-2 and C-10.
The relative configuration of 4 was inferred from the phase-sensitive NOESY experiment and established the same as compound 1. However, the experimental CD spectrum of 4 was similar to the calculated ECD curve generated for (1R,4S,5S,7S,10R)-4 and appeared as a mirror image of (1S,4R,5R,7R,10S)-4 (Fig. 4b), which indicated a reverse configuration of 4. Therefore, the absolute configuration of 4 was deduced to be 1R,4S,5S,7S,10R.
Compound 5 was identified as eucalyptin A by comparing its physical, MS, and NMR data with the literature values.10
Phloroglucinol-coupled sesquiterpenoids are mainly found in the genus Eucalyptus of the Myrtaceae family,11 and are here reported from the genus Rhodomyrtus for the first time. The skeleton of the phloroglucinol-coupled eudesmane reported previously was formed by a linkage to the C-7′ of the phloroglucinol motif.12 Rhodomyrtals A–C (1–3) represent a new skeleton of phloroglucinol coupled eudesmane through a C-12′ methylene; Rhodomyrtal D (4) contains a phloroglucinol unit of 3,5-dihydroxy-6,6-dimethyl-2-(3-methyl-1-oxobutyl)-2,4-cyclohexadien-1-one, which also coupled with an eudesmane moiety through a C-12′ methylene. This rare phloroglucinol unit has never been found in phloroglucinol-terpene adducts. We believe that these finding are of interest in the context of chemotaxonomy, plant biochemistry and synthetic chemical research.
The antibacterial activity of compounds 1–5 was evaluated against selected bacteria important in human infections as described previously.13 The results are shown in Table 1. Although compounds 1–5 were not active against the Gram-negative Pseudomonas aeruginosa, they showed relatively good antimicrobial activity against the two Gram-positive strains. Compounds 1 and 5 showed better activity than compounds 2, 3 and 4, with compound 5 being especially potent, having MIC and MBC values of 1.7 and 3.5 μg mL−1, respectively, against the biofilm producing strain, Staphylococcus epidermidis ATCC 35984.
| Microorganism | Compound | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| a Values shown are MIC (MBC) in μg mL−1. NA: not active at the maximum concentration tested. | |||||
| S. epidermidis ATCC 35984 | 2.4 (4.7) | 10 (20) | 15 (30) | 47.5 (95) | 1.7 (3.5) |
| S. aureus ATCC 29213 | 9.4 (18.8) | 40 (80) | 30 (60) | 47.5 (95) | 3.5 (13.8) |
| P. aeruginosa ATCC 27853 | NA | NA | NA | NA | NA |
Acknowledgements
This work was supported by the Wound Management Innovation CRC (established and supported under the Australian Government's Cooperative Research Centres Program) and the Cluster of Excellence: Engineering of Advanced Materials, FAU, Erlangen, Germany.Notes and references
- The Plant list home Page, http://www.theplantlist.org, accessed 14 October 2013.
- N. Snow, J. McFadden and J. P. Atwood, Austrobaileya, 2008, 7, 691–706 Search PubMed.
- D. Salni, M. V. Sargent, B. W. Skelton, I. Soediro, M. Sutisna, A. H. White and E. Yulinah, Aust. J. Chem., 2002, 55, 229–232 CrossRef CAS.
- A. Hiranrat and W. Mahabusarakam, Tetrahedron, 2008, 64, 11193–11197 CrossRef CAS PubMed.
- A. Hiranrat, W. Mahabusarakam, A. R. Carroll, S. Duffy and V. M. Avery, J. Org. Chem., 2012, 77, 680–683 CrossRef CAS PubMed.
- (a) J. Saising, A. Hiranrat, W. Mahabusarakam, M. Ongsakul and S. P. Voravuthikunchai, J. Health Sci., 2008, 54, 589–595 CrossRef CAS; (b) S. Limsuwan, E. N. Trip, T. R. H. M. Kouwen, S. Piersma, A. Hiranrat, W. Mahabusarakam, S. P. Voravuthikunchai, D. J. M. Van and O. Kayser, Phytomedicine, 2009, 16, 645–651 CrossRef CAS PubMed; (c) S. Limsuwan, A. Hesseling-Meinders, S. P. Voravuthikunchai, D. J. M. Van and O. Kayser, Phytomedicine, 2011, 18, 934–940 CrossRef CAS PubMed; (d) J. Saising, M. Ongsakul and S. P. Voravuthikunchai, J. Med. Microbiol., 2011, 60, 1793–1800 CrossRef PubMed; (e) W. Sianglum, P. Srimanote, W. Wonglumsom, K. Kittiniyom and S. P. Voravuthikunchai, PLoS One, 2011, 6, e16628 CAS; (f) M. Visutthi, P. Srimanote and S. P. Voravuthikunchai, J. Microbiol., 2011, 49, 956–964 CrossRef CAS PubMed; (g) S. Leejae, P. W. Taylor and S. P. Voravuthikunchai, J. Med. Microbiol., 2013, 62, 421–428 CrossRef CAS PubMed; (h) W. Sianglum, P. Srimanote, P. W. Taylor, H. Rosado and S. P. Voravuthikunchai, PLoS One, 2012, 7, e45744 CAS.
- G. Harden, Flora of New South Wales, Royal Botanic Gardens, Sydney, 2002, vol. 2, p. 229 Search PubMed.
- W. D. Crow, T. Osawa, D. M. Paton and R. R. Willing, Tetrahedron Lett., 1977, 12, 1073–1074 CrossRef.
- Z. Brkljača, K. Čondić-Jurkić, A.-S. Smith and D. M. Smith, J. Chem. Theory Comput., 2012, 8, 1694–1705 CrossRef.
- S. P. Yang, X. W. Zhang, J. Ai, L. S. Gan, J. B. Xu, Y. Wang, Z. S. Su, L. Wang, J. Ding, M. Y. Geng and J. M. Yue, J. Med. Chem., 2012, 55, 8183–8187 CrossRef CAS PubMed.
- J. F. Zeng and S. Q. Liu, in Chinese Flora Zhongguo Zhiwu Zhi, Science Press, Beijing, 1984, vol. 53, ch. 1, pp. 31–52 Search PubMed.
- K. Osawa and H. Yasuda, J. Nat. Prod., 1996, 59, 823–827 CrossRef CAS PubMed.
- Q. Shou, L. K. Banbury, D. E. Renshaw, E. H. Lambley, H. Mon, G. A. Macfarlane, H. J. Griesser, M. M. Heinrich and H. Wohlmuth, J. Nat. Prod., 2012, 75, 1612–1617 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, plant material, extraction and isolation, UV, IR, HRESIMS, 1D and 2D NMR spectra of rhodomyrtals A–D (1–4) as well as the theoretical methodology of CD calculations. See DOI: 10.1039/c4ra00154k |
| This journal is © The Royal Society of Chemistry 2014 |