Observing wetting behaviors of UV-curable liquid on nanostructured surfaces with sub-20 nm resolution†
Jie Biana,
Xinxin Fua,
Jing Hua,
Yushuang Cuia,
Zhiwei Lia,
Changsheng Yuan*a,
Haixiong Ge*a,
Wen-Di Li*b and
Yanfeng Chen*a
aDepartment of Materials Science and Engineering, College of Engineering and Applied Sciences, National Laboratory of Solid State Microstructures, Nanjing University, Nanjing, China. E-mail: csyuan@nju.edu.cn
bDepartment of Mechanical Engineering, University of Hong Kong, Pokfulam, Hong Kong, P. R. China. E-mail: liwd@hku.hk
First published on 21st March 2014
Abstract
In this work, the liquid acrylated materials were transferred onto the nanostructured surfaces by a transfer printing method. Further, they were frozen (solidified) by UV-light exposure. The morphology of the solidified liquid reflects the original liquid-state profile because the UV-curing shrinkage of the acrylated material was only a few percent in volume so that it would not largely alter the liquid morphology. The cured samples were then examined with high resolution SEM or AFM measurement. This proposed method allows for the observation of liquid behavior down to sub-20 nm scale with more subtle details such as nano-menisci between nanogrooves, and liquid bridges covering nanostructures. The wetting behavior of the liquid strongly depends on the surface properties and geometries of the underlying nanostructures. Experimental results agree with the prediction of a simple surface energy variation model. They indicate that macroscopic wetting behaviors of liquids are preserved as dimensions go down to 100 nm.
Introduction
The wetting behaviors of liquids on nanostructures have received continuous attention from fundamental sciences and applied technologies, including surface science,1–4 microfluidics,5,6 hydrodynamics,7,8 biomimetics,9–11 surface coating,12,13 anti-fogging14 and fog-harvesting,15,16 inkjet printing,17,18 biotechnology19,20 and thin-film lubrication.21,22 To better understand the wetting behaviors of the nanostructures, it is essential to look at the subtle features of the wetting morphologies with a close inspection at a local scale. The investigation of the interaction of the liquid with nanostructures at this scale is an emerging research area. It is still in its infancy and suffers from the lack of high spatial resolution observation techniques. Currently, atomic force microscopy (AFM),23–25 and environmental scanning electron microscopy (ESEM)26–28 are the most widely used techniques for observing liquid on nanostructured surfaces. Despite the recent progress in the named methods, many challenges still remain for both techniques. AFM has the capability of observing liquid morphologies down to nanoscale dimensions and operates in an ambient environment (air). However, the interaction between the AFM tip and the liquid can change the measured liquid profile and give rise to artifacts in the obtained image. Additionally, the time consuming AFM measurement limits its application for imaging volatile liquids, because the liquid morphology may vary in different ways during evaporation. On the other hand, the ESEM (or low-vacuum SEM) permits imaging of hydrated nonconductive samples and has been successfully applied to overcome the evaporation problem, but its resolution is in the micrometer range and is much lower than that on metal-coated samples. However, neither of these two methods can exhibit the real cross-section of the liquid filling into the nanostructure. Up to now, few existing methods can provide a direct observation for the interplay between liquid and structures down to the nanometer scale.Seemann et al.29 created liquid structures on micro-grooved surfaces by vapor condensation of polystyrene. The structures of the liquefied polymer were solidified by lowering the temperature below the glass transition temperature of polystyrene, and were then scanned by atomic force microscopy (AFM) without the aforementioned disadvantages. Krupenkin et al.30 dispensed UV polymerizable liquid droplets on nanostructures and solidified them by UV irradiation, then investigated their morphologies using SEM. Kusumaatmaja et al.31 created liquid monomer droplets on sub-micrometer-scale corrugated surfaces by microtransfer printing. The subsequent polymerization preserved the geometry of the liquid drop and enabled analysis afterward. Here, we present a combination of the methods mentioned above, to record the subtle features of liquid morphologies with sub-50 nm resolution based on UV-curable resins (solidifying liquid) on nanostructures. The wetting morphologies of the UV-curable liquid were created on nanostructures by a transfer printing method. These morphologies were instantaneously solidified by exposure to UV-light, and then were characterized by high resolution SEM or AFM measurement. The image of solidified liquid is a good indication of the liquid state, because the UV-curing shrinkage of the acrylated materials was only 6–7 percent in volume32 so that it would not largely change the original liquid morphology.
Experimental
Materials
A highly hydrophobic (HH) acrylated monomer (acrylated siloxane) and trichloro(1H,1H,2H,2H-perfluorooctyl)silane were purchased from Gelest. A low hydrophobic (LH) acrylated monomer (a hexafunctional urethane acrylate) was kindly provided by Sartomer. The photoinitiator, Irgacure 184, was obtained from Ciba. Also, a homemade silicon containing multifunctional acrylate composition was used as the UV-curable nanoimprint resist.Fabrication of nanostructured surfaces
The nanostructured surfaces were fabricated by UV-curing nanoimprint lithography. The UV-curable nanoimprint resist was spin-coated onto the silicon wafer at 2000 rpm for 40 s to get a 270 nm thickness smooth film and was imprinted by an imprint machine with combined thermal- and UV imprint capabilities from ImprintNano, China. The surfaces of the imprinted nanostructures were modified with a brief treatment of O2 plasma (30 W power, 5 sccm oxygen flow for 1 min) and then coated with a self-assembled monolayer of trichloro(1H,1H,2H,2H-perfluorooctyl)silane by vapor phase deposition in a closed dry container for 24 h.Transferring and solidifying UV-curable liquid on nanostructured surfaces
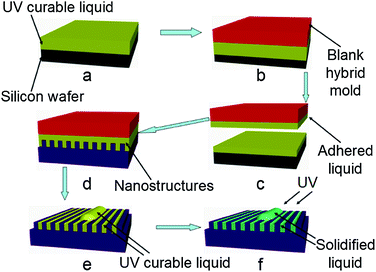
Both processes of transferring and solidifying UV-curable liquid on nanostructure surfaces are summarized in Fig. 1. Initially, the UV-curable liquid is spin-coated onto a silicon wafer at 2000 rpm for 40 s to form a 270 nm thick film (Fig. 1a). Next, a blank hybrid mold with a smooth surface is placed on the spin-coated UV-curable resist (Fig. 1b). After separation from the silicon wafer (Fig. 1c), the mold is placed on the nanostructure surface (Fig. 1d). The mold will spontaneously stick to the nanostructure surface by liquid capillary force without additional pressure. After the mold is removed from the nanostructure’s surface (Fig. 1e), the UV-curable liquid remains on the surface and is then cured by UV-light exposure (Fig. 1f).Measurement
SEM images were taken with a HITACHI S-4800 and a Carl Zeiss Supra 55 field-emission scanning electron microscope at 3.0 kV. AFM measurements were taken with a NT-MDT NSG20 in tapping mode. Contact angles were measured on a Dataphysics OCA30 CA system at ambient temperature.Results and discussion
Acrylate-based resins have been widely used in various UV-curable material systems as the backbone due to their great reactivity with a wide choice of acrylated monomers. Hence, by directly selecting or mixing a range of acrylate-based materials, one can formulate a UV-curable material with desirable properties. In this work, two different acrylated resins; a highly hydrophobic (HH) monomer (acrylated siloxane with a viscosity of 110 cps and contact angle (θ) of 58.8 ± 1.3° on a smooth hydrophobic surface formed by self-assembled monolayers (SAMs) of trichloro(1H,1H,2H,2H-perfluorooctyl)silane), and a low hydrophobic (LH) monomer (a hexafunctional urethane acrylate with a viscosity of 1600 cps and contact angle of 85.6 ± 1.8° on a smooth hydrophobic surface) were employed as the UV-curable liquids. Aside from the acrylated monomer, the UV-curable liquid consisted of 1.0 wt% photoinitiator (Irgacure 184) to the monomer and reactive diluent methyl acrylate for spin coating. Two different nanostructures were fabricated by employing UV-curing nanoimprint lithography and acrylated siloxane material.33 Fig. S1† of the ESI depicts grooves with 200 nm pitch and 140 nm depth, Fig. S2† of the ESI depicts square posts with 200 nm pitch and 110 nm height. In order to make the surface of the nanostructures hydrophobic, the surface was treated with SAMs of trichlorofluoroalkylsilane. Fig. S3† show that the silanization hardly changed the morphology of the 200 nm pitch nanogrooves, and the RMS roughness on the ridge of nanogrooves is 2.0 ± 0.5 nm. To investigate the wetting behavior on nanostructures at the micro and nanoscale, the bulk liquid must be transferred onto the nanostructured surface. In this work, the UV-curable liquid was formed on the nanostructured surface by a transfer printing method. The process of the transfer printing is illustrated in Fig. 1. A blank hybrid nanoimprint soft lithography mold34,35 consisting of a smooth crosslinked polymer film on an elastic poly(dimethylsiloxane) (PDMS) was employed as a carrier medium. First, a thin and uniform film of UV-curable liquid (resist) was spin-coated on a silicon wafer. Then, the mold was placed on the resist, covering the silicon wafer from one edge to the other edge. After a conformal contact between the mold and the silicon wafer was established, the mold was detached from the silicon wafer and some of the UV-curable liquid was adhered to the mold. The adhered liquid would ball up into droplets on the smooth surface of the blank mold by the minimization of surface energy. The mold was subsequently placed against the nanostructured surface. During this stage, the adhered liquid droplets were sandwiched between the blank mold and the substrate and would not only be pressed into the nanostructures but also planarized to extend into close contact with nearby droplets and merge into an integrated liquid film on the substrate.35 The UV-curable liquid was partially transferred onto the nanostructured surface after the mold was removed. The liquid would simultaneously turn to the thermodynamic equilibrium or metastable wetting morphologies. Finally, the UV-curable liquid on the nanostructures was instantaneously solidified by exposure to UV-light. Afterward, the solidified liquid is characterized by SEM and AFM.Fig. S5† shows the microscopic images of the uncured wetting morphologies and SEM images of the morphologies after photocrosslinking in an oxygen free environment for the HH and LH liquids. The various wetting morphologies, including microdroplets, liquid filaments and dewet regions, show almost no change during the UV curing process both for HH and LH liquid, hence the cured morphologies can be used to research the wetting behaviors for both liquids before crosslinking in this paper.
SEM images of the HH and LH UV-curable liquid droplets on the hydrophobic nanogrooves after curing are shown in the left and right columns of Fig. 2. The SEM images at low magnification show that the wetting behaviors of HH and LH liquid are significantly different. HH liquid resist droplets exhibit a consistent appearance over the sample surface, aside from some elongated micro-droplets randomly distributed on the surface, and a few dark filaments connecting the micro-droplets. However, the LH resist exhibited a bright-and-dark marble-like surface, scattered with elongated micro-droplets. The insets in Fig. 2a and b are the contact angle measurement of HH and LH liquid on the smooth hydrophobic surface, respectively. The HH liquid was more lyophilic (exhibited more wettability) than the LH liquid on the hydrophobic surface, therefore the HH liquid had a smaller contact angle. From the SEM images with higher magnification we could distinguish the differences between the wetting behaviors of the HH and LH liquid in the nanogrooves. Various wetting morphologies coexisted on the nanostructures for both liquids. In Fig. 2d it can be observed that the dark regions represent the nanogrooves filled with the liquid and the bright regions represent the empty nanogrooves with no liquid. The menisci of the liquid in the nanogrooves formed the boundary of the dark and bright regions in Fig. 2h. The micro-droplets were elongated along the groove direction and only resided on the dark region (Fig. 2d). As for the HH liquid, it was absorbed into the entire length of the grooves, therefore the SEM image showed a uniform contrast across the nanogroove surface, because there is only a slight variation in the projected height between the groove ridges and liquid-filled channels (Fig. 2c). The dark filaments were liquid bridges, which extended from micro-droplets and covered several ridges of the nanogrooves (Fig. 2e). If the images were continually zoomed in, more subtle features of the wetting morphologies could be observed. It was found that chains of isolated nanodroplets were located on top of the groove ridges for both liquids in Fig. 2f and g. These isolated nanodroplets also indicate that the liquid film did not cover the top of the ridge. The shapes of the nanodroplets were strongly influenced by the droplet size. If the contact diameter of the droplet was larger than the width of the groove ridge, the droplet was elongated along the ridge to exhibit an oval shape. Otherwise, if the contact diameter was less than the width of the ridge, then the droplet approached a spherical cap (Fig. 2h). It was also observed that the nanodroplet could adhere to the sidewall of the groove (Fig. 2g). In the right image of Fig. 2g, the smallest droplet that can be seen on the nanogrooves is 20 nm in diameter. There is a possibility for the existence of much smaller droplets on the groove surface; however it was difficult to distinguish them from the roughness of the surface (2.0 ± 0.5 nm RMS in ESI†).
To achieve a better understanding of the liquid behavior in the nanogrooves, the samples were carefully broken, and the cross sections of the wetting morphologies of the solidified liquids were captured by SEM. Fig. 3 shows the SEM images of the HH and LH liquid resists. The two liquids also showed different filling behaviors. For the HH liquid, the liquid filled all the channels, and some liquid bridges covered several ridges of the nanogrooves (Fig. 3a and c). However, for the LH liquid, only parts of the channels were filled and the others were empty (Fig. 3b and d). Liquid filled areas correspond to the dark regions and empty areas correspond to the bright regions on the top view of the SEM image (Fig. 2b). AFM measuring was done to confirm the SEM observations (Fig. 4a and b). The AFM results were in agreement with the SEM images. Series of isolated nanodroplets were found on top of the groove ridges (Fig. 4b), additionally, parts of the channels were filled with the liquid and others were empty (Fig. 3a). AFM produces deformed shapes when the size of the measured object is comparable to that of the AFM tip, thus the AFM image of the cross-sectional profile for the rectangular groove displayed a V-shaped bottom (Fig. 4c).
When the blank mold is inked with the UV-curable liquid from the spin-coated film, it is placed on the nanogrooves. The liquid resist would fill in the grooves and after removal of the mold, part of the liquid would remain in the nanogrooves. However, the remaining liquid resist would now become a non-equilibrium state. The interfacial free energies between the solid, the liquid, the vapor and the topography of the nanostructures would drive the liquid toward equilibrium or a metastable state. Quéré et al.36 systematically studied the wetting properties of liquids on micro and nanostructured surfaces. The complete wetting of any surface is only achieved when the θ = 0. This means that it is impossible to induce a wetting transition by texturing a solid. Due to the fact that the contact angles of both UV-curable liquids are larger than 0, both liquids formed droplets on the top surface of the nanogrooves. Since the top of the ridges was uncovered by the liquid film, the liquid behavior in the nanogroove array could be studied using a simplified model which considers liquid in a single isolated nanogroove. As the liquid progresses in a rectangular groove (Fig. S6†) with a width w, and a depth h, the surface energy variation dE arising from an apparent displacement dx of the liquid can be described as
| dE = (γSL − γSA)(2h + w)dx + γwdx | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where θ is the contact angle of the liquid on an ideal flat surface with the same chemical composition as the groove, we have
θ, where θ is the contact angle of the liquid on an ideal flat surface with the same chemical composition as the groove, we have
dE = −γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ(2h + w)dx + γwdx θ(2h + w)dx + γwdx
| (2) |
For dE = 0, the critical contact angle θc, for the favorable liquid progression can be expressed as
 | (3) |
The liquid progression is favorable (dE < 0) in the groove provided that the liquid contact angle, θ, is smaller than θc. In this case, the width and depth of the nanogrooves were around 100 nm and 140 nm, respectively, which were determined by the nanoimprinted mold and measured with SEM. The θc of the nanogroove was calculated to be 74.7°. The contact angles of the HH and LH liquids on the flat surface coated with trichlorofluoroalkylsilane were 58.8 ± 1.3° (<θc) and 85.6 ± 1.8° (>θc), respectively. The SEM observation was in agreement with the predicted results. The HH liquid filled the full length of the nanogroove while the LH liquid partially receded from the groove. Besides the thermodynamic equilibrium states, metastable states were also achieved, HH liquid bridges spanned several ridges of the nanogrooves because the sharp edges of the ridges represented energy barriers for the retraction of the liquid film and locally pinned the filament to the upper edges of the grooves.
Similar experiments were done on a surface decorated with square nanoposts. Fig. 5a and b show the wetting behaviors of the HH and LH liquids on the nanoposts coated with trichlorofluoroalkylsilane, respectively. The liquid film should also recede to ball up on the flat top of the nanoposts because their contact angles were much larger than 0. Their wetting properties could still be interpreted by Quéré's model37 written as
| dE = (γSL − γSA)(r − φS)dx + γ(1 − φS)dx | (4) |
 | (5) |
For the square nanoposts with sizes of 110 nm, pitch of 200 nm and height of 110 nm, we obtained r = 2.2 and φS = 30%. Its θc was calculated to be 68.4°. Since the contact angle of the LH liquid was larger than 68.4°, the liquid exhibited dewetting properties on the nanoposts surface. It receded through the trenches of the nanoposts and balled up to micro-droplets (Fig. 5b). The shape of the micro-droplets was deformed by the pinning effect of the nanoposts and partially conformed to the nanoposts array (Fig. 5d). In addition, the liquid film left on top of the isolated nanopost also balled up to a nanodroplet (Fig. 5d). The HH liquid showed wetting properties on the nanoposts. Almost all of the trenches of the nanoposts were filled by the liquid because its contact angle was smaller than 68.4°. Unlike the LH liquid, which balled up to nanodroplets, the HH liquid spread out on the top facet of the nanopost. This might be attributed to the very small surface area of the top of a single nanopost and stronger pinning effects at the four edges of the nanopost compared to the LH liquid, which locally hindered the liquid film from receding to nanodroplets. It was observed that local dewetting (nanogaps) happened at several parts of the nanoposts surface, which might be caused by the defects of the SAM coating of trichlorofluoroalkylsilane (Fig. 5a and c).
A series of square arrays of cylindrical silicon nanoposts with various aspect ratios (ratio of the post height to the pitch) were fabricated by nanoimprint lithography and reactive ion etching, and then coated with a SAM layer of trichlorofluoroalkylsilane (Fig. S8†). Post diameter d and pitch p were almost fixed (about 200 and 400 nm, respectively) from the imprint mold, while the height h was varied by the etching time of RIE. In the case of cylindrical posts, we had r = 1 + πdh/p2 and φS = πd2/4p2. Fig. 6 shows the wetting behaviors of the HH liquid on the square arrays of cylindrical silicon nanoposts with various aspect ratios. For small aspect ratio posts, it was observed in Fig. 6a that almost all the liquid was drained from the cavities between the nanoposts and balled up to micro-droplets. As the aspect ratio increased, the liquid morphologies gradually transited from a drainage state to an imbibitional state (Fig. 6b and c). This is because the critical contact angle increased with increasing post aspect ratio. Once the contact angle of the liquid was less than the critical contact angle, dE would become less than zero, which would drive the liquid imbibed into cavities between the nanoposts. If the volume of the liquid was smaller than that of the cavities, the liquid could not completely wet all the cavities, while it would also assemble in the cavities, just as the liquid film balling up droplets on the flat surface due to its contact angle >0 (Fig. 6d). More subtle details of the liquid behavior could be obtained from the observation of the cross-section of the nanoposts arrays (Fig. 6e and f). The liquid film would conform the shape of the textures in incompletely imbibitional state on 110 nm depth nanoposts surface (Fig. 6e) and the meniscus at the centre of liquid film was higher than the one at the edge for 250 nm depth nanoposts (Fig. 6f). The liquid remained on top of the circle nanopost, balled up to nanodroplets (Fig. 6f) and the contact angle was approximately the same as that of its macroscopic droplet (Fig. S9†). The observation was in good agreement with the prediction of a simple surface energy variation model.
Conclusions
We developed a novel method for studying the wetting behavior of liquids at the micro and nanoscale via transferring and solidifying UV-curable liquid on a nanostructured surface. The solidified samples were applicable to high resolution SEM and AFM measurement. This method was able to extend the nanoscale observation of liquid down to sub-20 nm. The wetting behaviors of the liquid strongly relied on the surface properties and geometries of the nanostructures. The results indicated that macroscopic wetting behavior of liquid is preserved all the way down to 100 nm.Acknowledgements
This work was jointly supported by the National Nature Science Foundation of China (Grant no. 91023014 and 61306123), the National Basic Research Program of China (973 Program) (Grant no. 2013cb632702), the Priority Academic Program Development of Jiangsu Higher Education Institutions and RFDP, and the New Century Excellent Talent Project of the Ministry of Education of China (Grant no. NCET-10-0455).Notes and references
- R. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Nature, 1945, 155, 21 CrossRef CAS.
- A. Lafuma and D. Quéré, Nat. Mater., 2003, 2, 457 CrossRef CAS PubMed.
- Á. Detrich, M. Nyári, E. Volentiru and Z. Hórvölgvi, Mater. Chem. Phys., 2013, 140, 602 CrossRef PubMed.
- S. Jeon, V. Malyarchuk, J. O. White and J. A. Rogers, Nano Lett., 2005, 5, 1351 CrossRef CAS.
- Q. Xia, K. J. Morton, R. H. Austin and S. Y. Chou, Nano Lett., 2008, 8, 3830 CrossRef CAS PubMed.
- P. Kim, H. Y. Kim, J. K. Kim, G. Reiter and K. Y. Suh, Lab Chip, 2009, 9, 3255 RSC.
- D. J. Lee, K. Y. Cho, S. Jang, Y. S. Song and J. R. Youn, Langmuir, 2012, 28, 10488 CrossRef CAS PubMed.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1 CrossRef CAS.
- X. Gao, X. Yan, X. Yao, L. Xu, K. Zhang, J. Zhang, B. Yang and L. Jiang, Adv. Mater., 2007, 19, 2213 CrossRef CAS.
- X. Gao and L. Jiang, Nature, 2004, 432, 36 CrossRef CAS PubMed.
- W. K. Cho, S. Park, S. Jon and I. S. Choi, Nanotechnology, 2007, 18, 395602 CrossRef PubMed.
- S. C. Thickett, C. Neto and A. T. Harris, Adv. Mater., 2011, 23, 3718 CrossRef CAS PubMed.
- K. K. S. Lau, J. Bico, K. B. K. Teo, M. Chowalla, G. A. J. Amaratunga, W. I. Milne, G. H. McKinley and K. K. Gleason, Nano Lett., 2003, 3, 1701 CrossRef CAS.
- H. Bai, X. Tian, Y. Zheng, J. Ju, Y. Zhao and L. Jiang, Adv. Mater., 2010, 22, 5521 CrossRef CAS PubMed.
- S. P. R. Kobaku, A. K. Kota, D. H. Lee, J. M. Mabry and A. Tuteja, Angew. Chem., Int. Ed., 2012, 51, 10109 CrossRef CAS PubMed.
- J. Z. Wang, Z. H. Zheng, H. W. Li, W. T. S. Huck and H. Sirringhaus, Nat. Mater., 2004, 3, 171 CrossRef CAS PubMed.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 290, 2123 CrossRef CAS.
- K. F. Lei, Recent Pat. Nanotechnol., 2013, 7, 81 CrossRef CAS.
- I. Platzman, C. A. Muth, C. Lee-Thedieck, D. Pallarola, R. Atanasova, I. Louban, E. Altrock and J. P. Spatz, RSC Adv., 2013, 3, 13293 RSC.
- U. Bauer and W. Federle, Plant Signaling Behav., 2009, 4, 1019 CrossRef.
- T. S. Wong, S. H. Kang, S. K. Y. Tang, E. J. Smythe, B. D. Hatton, A. Grinthal and J. Aizenberg, Nature, 2011, 477, 443 CrossRef CAS PubMed.
- P. K. Hansma, G. Schitter, G. E. Fantner and C. Prater, Science, 2006, 314, 601 CrossRef CAS PubMed.
- R. Wang and M. Kido, Surf. Interface Anal., 2005, 37, 1105 CrossRef CAS.
- A. Méndez-Vilas, A. B. Jódar-Reyes and M. L. González-Martín, Small, 2009, 5, 1366 CrossRef PubMed.
- D. J. Stokes, Adv. Eng. Mater., 2001, 3, 126 CrossRef.
- Y. C. Jung and B. Bhushan, J. Microsc., 2008, 229, 127 CrossRef CAS PubMed.
- K. Rykaczewski and J. H. J. Scott, ACS Nano, 2009, 7, 5962 Search PubMed.
- R. Seemann, M. Brinkmann, E. J. Kramer, F. F. Lange and R. Lipowsky, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 1848 CrossRef CAS PubMed.
- T. N. Krupenkin, J. A. Taylor, T. M. Schneider and S. Yang, Langmuir, 2004, 20, 3824 CrossRef CAS.
- H. Kusumaatmaja, R. J. Vrancken, C. W. M. Bastiaansen and J. M. Yeomans, Langmuir, 2008, 24, 7299 CrossRef CAS PubMed.
- X. Hu, T. Yang, R. Gu, Y. Cui, C. Yuan, H. Ge, W. Wu, W. Li and Y. Chen, J. Mater. Chem. C, 2014, 2, 1836 RSC.
- H. Ge, W. Wu, Z. Li, G. Y. Jung, D. Olynick, Y. Chen, J. A. Liddle, S. Y. Wang and R. S. Williams, Nano Lett., 2005, 5, 179 CrossRef CAS PubMed.
- Z. Li, Y. Gu, L. Wang, H. Ge, W. Wu, Q. Xia, C. Yuan, Y. Chen, B. Cui and R. S. Williams, Nano Lett., 2009, 9, 2306 CrossRef CAS PubMed.
- Y. Shen, L. Yao, Z. Li, J. Kou, Y. Cui, J. Bian, C. Yuan, H. Ge, W. Li, W. Wu and Y. Chen, Nanotechnology., 2013, 24, 465304 CrossRef PubMed.
- D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 71 CrossRef.
- J. Bico, C. Tordeux and D. Quéré, Europhys. Lett., 2001, 55, 214 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of the nanostructures and solidified liquid, AFM images, contact angle images of liquid are presented here. See DOI: 10.1039/c4ra00088a |
| This journal is © The Royal Society of Chemistry 2014 |