A new nano-sized calcium hydroxide photocatalytic material for the photodegradation of organic dyes
Shujuan Zhang*
College of Science, Tianjin University of Science & Technology, Tianjin, China. E-mail: songlmnk@sohu.com
First published on 13th March 2014
Abstract
Nano-sized calcium hydroxide (Ca(OH)2) photocatalysts were synthesized by a precipitation method. The as-prepared Ca(OH)2 samples were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), UV-vis absorption spectroscopy and photoluminescence spectroscopy (PL). The as-obtained Ca(OH)2 samples showed excellent photocatalytic degradation activities against methylene blue (MB) aqueous solution under visible light radiation. Dependence of the activity on various experimental conditions was investigated, and the results suggested promising utilization of the Ca(OH)2 photocatalysts. In addition, the photocatalytic degradation of MB was well fitted to pseudo-first-order kinetics. Therefore, MB was photocatalytically degraded over Ca(OH)2 through indirect dye photosensitization.
Introduction
In the past decade, organic pollutants, which lead to environmental contamination that seriously endangers the entire world, can be effectively removed from wastewater by semiconductor photocatalysis materials with high efficiency, nontoxicity, photochemical stability and low cost. Of the reported semiconductors, oxides (TiO2, ZnO, WO3),1–3 sulfides (MoS2, ZnS, In2S3),4,5 and halides (AgCl, BiOI)6,7 have been utilized as photocatalysts to oxidize organic pollutants.8–11 However, their practical applications are limited by the rapid recombination of photogenerated electron–hole pairs, low quantum yields in reactions, and extremely low responses to visible light. Therefore, it is critical to develop novel photocatalysts. In addition to the above-mentioned photocatalysts, metal hydroxide photocatalysts also showed good photocatalytic properties. For example, Li et al. reported both pure and modified In(OH)3 had excellent photocatalytic activities.12–14 Ca(OH)2 has been widely used as additive for lubricants and dentistry.15,16 However, Ca(OH)2 nanomaterials have never been reported as photocatalysts so far. Therefore, we prepared Ca(OH)2 nanoparticles by an original method in this study. The structure and composition of samples were characterized systematically. The nano-sized Ca(OH)2 exhibited strong photodegradation activities against MB solution under both UV and visible lights. Meanwhile, under visible light radiation, such photocatalysis was a surface-related photosensitization process. The effects of basic catalyst on the photocatalytic reaction of MB were analyzed in detail, providing valuable references for other metal hydroxide photocatalysts.Experimental
Synthesis of Ca(OH)2 samples
Anhydrous calcium chloride (CaCl2, 10 g) was dissolved in 20 mL of deionized water under stirring. A transparent clear solution was obtained (solution 1). NaOH (7.21 g) was also dissolved in 20 mL of deionized water under stirring to acquire solution 2. Solution 1 was dropwisely added to solution 2 under vigorous stirring at room temperature. The solution gradually became turbid and produced numerous white precipitates. The mixture was continuously stirred for 30 min until Ca(OH)2 was completely precipitated. The resultant mixture was filtrated and washed with deionized water and ethanol. The as-obtained Ca(OH)2 precipitates were air-dried at 120 °C for 3 h.Characterization of samples
The phase and composition of the samples were analyzed by X-ray diffraction (XRD, Rigaku D/max 2500, λ = 1.5406 Å, 40 kV, 40 mA). The size and morphologies were observed by a field emission scanning electron microscope (FESEM, Hitachi, S-4800) and a transmission electron microscope (TEM, Hitachi, H-7650). The accelerating voltages of FESEM and TEM were 10 kV and 100 kV, respectively. The UV-vis absorption spectra were recorded on an HP8453 spectrophotometer equipped with an integration sphere using BaSO4 as the reference. Photoluminescence spectra (PL) were measured using a Cary Eclipse photoluminescence analyzer.Photocatalytic activity measurement
In a typical experiment, the photocatalytic activity of the samples was measured by the photodegradation of MB solution (10 mg L−1) in a reactor (100 mL) with 300 mg samples under visible light irradiation. A 300 W Xe lamp was used directly for the photocatalytic reaction, while a 400 nm cutoff filter was employed for visible-light photocatalysis to remove UV light. All the mixtures were stirred at room temperature in dark for 30 min to reach adsorption equilibrium before irradiation, and air was continuously bubbled in the solution to provide O2 that auxiliarily photodegraded MB molecules over Ca(OH)2. Hydroxyl and superoxide radicals are oxidation species in the photocatalytic system, and superoxide radicals are generated in the presence of O2. At a defined time interval, the mixture of Ca(OH)2 and dye solution was extracted and centrifuged to remove Ca(OH)2 particles. The remaining MB solution was analyzed by using a UV-vis spectrophotometer at 665 nm. The removal ratio was calculated by (C0 − C)/C0, where C is the residual concentration after reaction, and C0 is the original concentration after adsorption equilibrium.Measurement of the point of zero charge for Ca(OH)2
The point of zero charge (PZC) of Ca(OH)2 was measured by the salt titrimetric method. Ca(OH)2 (0.5 g) and 1, 2.5, 5, 7.5, 10, 12.5, 15, 17.5 mL of 0.1 mol L−1 NaOH were added to different beakers, respectively. A certain amount of deionized water was added to the beakers to reach a 20 mL total volume. After stirring for 1 h, these solutions were equilibrated for 4 days at room temperature. The pH of the suspension was measured after stirring for 1 h per day. Then 0.5 mL of 2 mol L−1 NaCl solutions were added to the above mixtures, respectively. The pH was measured after stirring for 3 h (referred to as pH 1). A curve was plotted with the pH abscissa and ΔpH ordinate (ΔpH = pH − pH1), based on which the PZC of Ca(OH)2 was calculated.Hydroxyl radicals measurement
The formation of OH˙ radicals under visible-light irradiation was detected by PL. Samples (5 mg) were added to 80 mL of aqueous solution (0.01 mol L−1 NaOH, 3 mmol L−1 terephthalic acid), and stirred for 30 min in dark. At a defined time interval (5 min), the concentration of solution was determined by PL under visible-light irradiation (excited at 325 nm).Results and discussion
Characterization of photocatalysts
The XRD patterns of the as-prepared sample are exhibited in Fig. 1. Main diffraction peaks are in good agreement with the JCPDS card no. 87-0674 of a typical hexagonal-phase Ca(OH)2 crystal (a = b = 3.589 Å, c = 4.911 Å, space group P3m1[164]), which only differ in the stronger intensity of (001) diffraction peak than that of (101), indicating that Ca(OH)2 particles probably prefer to grow along the c-axis. Meanwhile, the dominant (001) facets and the facades of the particles are enclosed by (001) facets. Besides, trace CaCO3 impurities that originated from the reaction of Ca(OH)2 and CO2 in air were found. The strongest (001) peak was used to calculate the crystal sizes of the samples by the Scherrer equation, and the mean size is 38 nm.As evidenced by the TEM and SEM images of the as-made samples (Fig. 2), the morphology is not monotonous. The irregular nano-sized Ca(OH)2 particles (Fig. 2a) do not agglomerate because they have been ultrasonically dispersed in ethanol. However, single particles consist of these multiple tiny particles. The maximum and the minimum sizes are 75 nm and 20 nm respectively, and the mean size is 35 nm. The as-prepared particles were further measured by SEM in order to investigate the surface morphology. The particles of Ca(OH)2 in Fig. 2b seriously reunite so that they become larger obviously, which may affect the exposure of surface active sites, thereby affecting the photocatalytic activity.
Fig. 3A shows the UV-vis absorption spectrum of the as-made Ca(OH)2. There is an apparent, intense absorption edge in the UV light region, and a weak absorption edge can also be discerned in the visible light range. The band gap energy of Ca(OH)2 is estimated as 5.7 eV (Fig. 3B), which indicates that the as-synthesized Ca(OH)2 nanoparticles can hardly be excited by visible light. The valence band of the as-made Ca(OH)2 was measured by XPS, and the peak corresponds to O2 p states in Ca(OH)2. It has been previously reported that the binding energy of the onset edge of the O2 p peak has an energy gap (Evf) between the valence-band maximum and Fermi level (Ef).17 The energy gap (Evf) of Ca(OH)2 is approximately 2.5 eV (Fig. 3C), so the energy gap of the conduction-band bottom of Ca(OH)2 is estimated as 3.2 eV by subtracting 2.50 eV from the band gap of Ca(OH)2 (5.7 eV). The Fermi level (Ef), the conduction and the valence bands of Ca(OH)2 are illustrated in Fig. 3D.
 | ||
| Fig. 3 UV-vis absorption spectra (A and B), valence-band XPS spectra (C), and position of conduction band (CB) and valence band (VB) (D) of the as-synthesized Ca(OH)2. | ||
Fig. 4a and b show the PL emission spectra of the as-prepared and commercial Ca(OH)2 samples (about 51 nm for the as-prepared Ca(OH)2; about 0.3 μm for commercial Ca(OH)2), respectively. Only one peak at about 435 nm is observed for the two samples, which is assigned to the transitions band of Ca(OH)2. According to a previous literature,18 photo-generated electrons in the conduction band that fell into oxygen vacancies through a non-radiation process then recombined with photo-generated holes in the valence band, which thus emitted fluorescence. Since there was only a weak PL emission of Ca(OH)2 (Fig. 4a and b), the charge carriers merely recombined slightly. Obviously, the PL peak intensity of the as-prepared Ca(OH)2 is lower than that of commercial Ca(OH)2, inferring that nanosized Ca(OH)2 exhibited enhanced photocatalytic activity by inhibiting the recombination of charge carriers.
Photocatalytic activity of photocatalysts
According to previous literatures,19,20 we measured the pH values of the mixture of Ca(OH)2 at different concentrations and 10 mg L−1 MB−1 solution stirred for 1 h (Table. 1). The pH values of the mixed solution were higher than 12. Fig. 5A shows the UV-visible spectra of the mixed solution. The two absorption peaks at 290 and 665 nm can be assigned to the characteristic structure of MB molecules. The maximum absorption peaks at 665 nm were significantly lower because some MB molecules had been converted into methylene violet (MV) under alkaline conditions.19,20 However, the absorbance of the degraded solution remained constant (close to zero) with increasing reaction time (Fig. 5B), which may be attributed to further degradation of remnant dye molecules under natural light radiation owing to trace Ca(OH)2 in solution. Hence, MB and MV molecules were degraded over Ca(OH)2 under light radiation. In other words, the decay of MB did not affect the photodegradation rate of MB over Ca(OH)2 obviously. Since MB is a type II photosensitizing dye,21 a blank experiment was also carried out to investigate the effect of MB on photolysis (Fig. 5C). The photolysis rate ranged from 6.86% to 10.5% without Ca(OH)2 after 10 min of visible light radiation, and the values remained constant thereafter. Therefore, the photolysis of MB molecules barely affected photodegradation. Total organic carbon (TOC) value in the photodegradation of MB over Ca(OH)2 was also investigated. The concentrations of MB and Ca(OH)2 are 10 mg L−1 and 3 g L−1, respectively. The TOC value of the original MB solution (10 mg L−1) is approximately 5.181 mg L−1, and that of MB solution is around 3.951 mg L−1 after 30 min. Accordingly, MB molecules had been photodegraded in the photocatalytic reaction. In addition, Ksp[Ca(OH)2] of 5.5 × 10−6 may affect the dissolution of particles in aqueous solution. Therefore, the shape and size of particles after irradiation were analyzed by TEM. The maximum and the minimum sizes were 29 nm and 15 nm respectively, and the mean size was 19 nm (Fig. 5D). These sizes were significantly reduced after irradiation in comparison with those of the fresh Ca(OH)2 particles (Fig. 2a) due to low solubility and insufficient stirring.| Solution | MB | MB + 0.1 mol L−1 NaOH | MB + 2.25 g L−1 Ca(OH)2 | MB + 3.0 g L−1 Ca(OH)2 | MB + 3.75 g L−1 Ca(OH)2 |
|---|---|---|---|---|---|
| pH | 7.0 | 12.72 | 12.22 | 12.41 | 12.44 |
Various amounts of Ca(OH)2 (2.25 to 3.75 g L−1) were added to find out the one that led to the highest photodegradation activity. Fig. 5C shows that the adsorption capacities of the three reactions remain constant after 20 min, suggesting that the adsorption in dark had reached equilibrium. The highest adsorption capacities of the three reactions are 10.8%, 9.4% and 12.8% respectively, which may be associated with the surface charge of Ca(OH)2 colloidal particles as well as the surface group and charge of MB in water. The PZC of Ca(OH)2 was measured by the salt titrimetric method. Fig. 5E shows that the PZC of Ca(OH)2 is 12.9. The pH increases slightly from 12.73 to 12.83 during photocatalysis. However, the pH is lower than the PZC of Ca(OH)2 in water, indicating the surface charge of Ca(OH)2 colloidal particles is positive under our experimental conditions. MB is also positively charged owing to the detaching of Cl− in water. Thus, the high adsorption of MB over Ca(OH)2 can be attributed to the large number of –NH2 group in MB molecules rather than the surface charge. The high MB adsorption capacity over Ca(OH)2 facilitated the degradation because the catalytic reaction occurred on the surface. However, since the surface of Ca(OH)2 was not blue after the reaction, the MB molecules adsorbed on the surface had also been degraded despite of the high adsorption capacity. Fig. 5C also reveals that the photodegradation ratio is the highest when 3.75 g L−1 Ca(OH)2 was added to the solution. However, the photocatalytic activity was not significantly improved after 30 min compared to that of 3 g L−1 catalyst. Therefore, 3 g L−1 Ca(OH)2 was selected to perform the photocatalytic reaction.
Moreover, MB solutions at initial concentrations ranging from 5 to 20 mg L−1 (3 g L−1 Ca(OH)2) were used. Similarly, the four reactions reached adsorption equilibria after 30 min of stirring in dark (Fig. 5F). As shown in Fig. 5F, the photodegradation ratios are 95, 95, 83 and 57% after 30 min, respectively. The photodegradation activities are highest in the presence of 5 and 10 mg L−1 Ca(OH)2, and the degradation efficiency decreases with increasing concentration of MB. The ln(C0/C) values of MB are linearly correlated with the irradiation time, indicating the photodegradation is a pseudo-first-order reaction (Fig. 5G). The fitting parameters of MB photodegradation kinetics are listed in Table. 2. The rate constants of photodegradation are calculated as 0.093, 0.077, 0.06 and 0.02 min−1, revealing a rising photocatalytic activity order with decreasing initial MB concentration.
| Ca(OH)2 Concentration | k (min−1) | R2 |
|---|---|---|
| 5 mg L−1 | 0.093 | 0.9962 |
| 10 mg L−1 | 0.077 | 0.9914 |
| 15 mg L−1 | 0.06 | 0.9970 |
| 20 mg L−1 | 0.02 | 0.9683 |
Hydroxyl radicals (˙OH) and photocatalytic mechanism
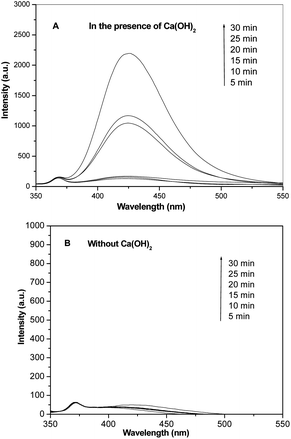
˙OH radicals after visible light irradiation were examined by PL to clarify the active species during the photodegradation of Ca(OH)2. Fig. 6 exhibits the dependence of the PL spectrum of terephthalic acid solution on irradiation time. The PL intensity at 425 nm gradually rose with increasing irradiation time in the presence of Ca(OH)2 (Fig. 6A), indicating that fluorescence was enhanced by the reaction of terephthalic acid with ˙OH produced by Ca(OH)2 photocatalysis. Fig. 6B shows that water produces ˙OH under visible light irradiation without Ca(OH)2, and low intensity at 425 nm can be observed, indicating that the increase of ˙OH in water resulted from Ca(OH)2.Fig. 3 exhibits that Ca(OH)2 particles cannot be excited by visible light because of a wide gap band (5.7 eV). Therefore, the photocatalysis of Ca(OH)2 resulted from indirect dye photosensitization rather than photoinduced charge carriers. Previous studies have reported that indirect dye photosensitization degradation included photoexcitation of dye molecules, injection of photoexcited electrons into the conduction band of Ca(OH)2, and capture of the injected electrons by surface-adsorbed O2 molecules that produces active species (O2−˙, OH˙, etc.) for the degradation of organic compounds.22,23 The valence band of Ca(OH)2 does not participate in the photoreaction during photosensitization. Notably, dye molecules directly interact with the semiconductor during the injection of photoexcited electrons into the conduction band of Ca(OH)2. Therefore, the adsorption of a large number of MB molecules on surface of Ca(OH)2 particles (Fig. 5C and D) is conducive to photosensitization, which boosts the photocatalytic activity of Ca(OH)2.
Conclusion
In short, Ca(OH)2 nano-photocatalytic materials have been prepared by a traditional method. The average size of the as-obtained Ca(OH)2 particles was about 52 nm. The as-prepared Ca(OH)2 particles absorbed intensely in the visible region. The decreased TOC values of MB solution after a photocatalytic reaction suggested complete photodegradation activities over Ca(OH)2. The MB solution was subjected to slight pH changes during photodegradation, which did not affect the photocatalytic activities obviously. The high adsorption of MB over Ca(OH)2 can be attributed to the –NH2 group in MB molecules instead of their surface charge. The Ca(OH)2 samples were of superior photodegradation activity against MB. Besides, the photocatalytic degradation of MB was well fitted to pseudo-first-order kinetics, and the photocatalytic degradation of MB over Ca(OH)2 was an indirect dye photosensitization process.References
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed
.
- S. U. M. Khan, M. Al-Shahry and W. B. Ingler, Science, 2002, 297, 2243 CrossRef CAS PubMed
.
- I. Justicia, P. Ordejon and G. Canto, Adv. Mater., 2002, 14, 1399 CrossRef CAS
.
- Y. Zhang and Y. D. Li, J. Phys. Chem. B, 2004, 108, 17805 CrossRef CAS
.
- Q. R. Zhao, Y. Xie, Z. G. Zhang and X. Bai, Cryst. Growth Des., 2007, 7, 153–158 CAS
.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai and M. H. Whangbo, Inorg. Chem., 2009, 48, 10697 CrossRef CAS PubMed
.
- P. Wang, B. B. Huang, X. Y. Qin, H. Jin, Y. Dai, Z. Y. Wang, J. Y. Wei, J. Zhan, S. Y. Wang, J. P. Wang and M. H. Whangbo, Chem.–Eur. J., 2009, 15, 1821 CrossRef CAS PubMed
.
- G. C. Xi, J. H. Ye, Q. Ma, N. Su, H. Bai and C. I. Wang, J. Am. Chem. Soc., 2012, 134, 6508 CrossRef CAS PubMed
.
- L. Y. Yang, S. Y. Dong, J. H. Sun, J. L. Feng, Q. H. Wu and S. P. Sun, J. Hazard. Mater., 2010, 179, 438 CrossRef CAS PubMed
.
- Z. G. Yi, J. h. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. S. Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559 CrossRef CAS PubMed
.
- Y. P. Bi, S. X. Ouyang, N. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS PubMed
.
- S. W. Hu, J. Zhu, L. Wu, X. X. Wang, P. Liu, Y. F. Zhang and Z. H. Li, J. Phys. Chem. C, 2011, 115, 460 CAS
.
- Z. H. Li, H. Dong, Y. F. Zhang, T. T. Dong, X. X. Wang, J. Q. Li and X. Z. Fu, J. Phys. Chem. C, 2008, 112, 16046 CAS
.
- Z. H. Li, T. T. Dong, Y. F. Zhang, L. Wu, J. Q. Li, X. X. Wang and X. Z. Fu, J. Phys. Chem. C, 2007, 111, 4727–4733 CAS
.
- B. Delfort, M. Born, A. Chivé and L. Barré, J. Colloid Interface Sci., 1997, 189, 151 CrossRef CAS
.
- H. G. Shin, H. Kim, Y. N. Kim and H. S. Lee, Curr. Appl. Phys., 2009, 9, S276 CrossRef
.
- Y. Cao, T. He, Y. Chen and Y. Cao, J. Phys. Chem. C, 2010, 114, 3627 CAS
.
- D. Li, H. Haneda, S. Hishita and N. Ohashi, Chem. Mater., 2005, 17, 2588 CrossRef CAS
.
- A. Mills, D. Hazafy, J. Parkinson, T. Tuttle and M. G. Hutchings, Dyes Pigm., 2011, 88, 149 CrossRef CAS
.
- L. Adamcíková, K. Pavlíková and P. Seveík, React. Kinet. Catal. Lett., 2000, 69, 91 CrossRef
.
- F. Wilkinson, W. Phillip Hellman and A. B. Ross, J. Phys. Chem. Ref. Data, 1993, 22, 113 CrossRef CAS
.
- J. C. Zhao, C. C. Chen and W. H. Ma, Top. Catal., 2005, 35, 269 CrossRef CAS
.
- C. C. Chen, J. C. Zhao and W. H. Ma, Chem. Soc. Rev., 2010, 39, 4206 RSC
.
| This journal is © The Royal Society of Chemistry 2014 |