A thermally switchable chromatographic material for selective capture and rapid release of proteins and nucleotides†
Zongjian Liu‡
ab,
Rui Su‡ac,
Xiao Lianga,
Yanli Lianga,
Yulin Deng*a,
Yujuan Lia and
Rongji Dai*a
aSchool of Life Science, Beijing Institute of Technology, Beijing 100081, China. E-mail: deng@bit.edu.cn; dairongji@bit.edu.cn; Fax: +86 10 6846 7208; Tel: +86 10 6894 9331
bChina-America Institute of Neuroscience, Luhe teaching hospital, Capital Medical University, Beijing 101100, China
cSchool of Chemical Engineering and Environment, North University of China, Taiyuan 030051, China
First published on 21st March 2014
Abstract
We report a switchable chromatographic material for the selective capture-release of proteins with simpler adjustment of the temperature instead of the conventional need to change the mobile phase. This novel strategy greatly increased the number of LC/MS/MS-identified proteins from human serum, as well as their scores and coverage ratios.
Chromatographic techniques play a significant role in the separation and purification of biomolecules such as peptides and proteins.1 The separation of biomolecules such as proteins has distinct challenges, given their considerable complexity and wide range of abundance. Removal of highly abundant proteins from complex biological samples has been shown to greatly increase the number of identified proteins using proteomics approaches. Selective enrichment of low abundant proteins such as phosphoproteins2 and glycoproteins3 is still an indispensable step prior to MS analysis. There are various affinity chromatographic materials such as immobilized metal affinity chromatography, boronate affinity chromatography, antibodies and dye ligands affinity chromatography for depletion/enrichment of target proteins in proteomics study.1–4 However, the switching of mobile phases is required for the release of adsorbed proteins using the conventional materials. Often, the harsh denaturants and/or variable salt concentrations, pH values are used. Usually, a relatively long equilibration time is required after changing the mobile phases. Therefore, changing the composition of mobile phase is well-known to be tedious. Moreover, with rapid developments in proteomics, there is a significant increase in the demand for highly selective chromatographic materials for the separation and enrichment of low abundance proteins from complex biological matrices.
Thermally responsive materials have recently sparked much research interest.5 Poly(N-isopropylacrylamide) (PNIPAAm) is the most extensively studied thermoresponsive polymer with a low critical solution temperature (LCST) of 32 °C and it exhibits sharp changes in molecular conformation and hydrophilic/hydrophobic properties under thermal stimuli.6 These properties have been exploited to create thermoresponsive chromatographic materials for the separation of steroids, amino acids, peptides, proteins, and other compounds.7,8 The introduction of cationic monomers such as (N,N-dimethylamino) propylacrylamide (DMAPAAm) or 2-(dimethylamino)ethylmethacrylate (DMAEMA), facilitated the separation of ionic compounds.9 Thermosensitivity was usually evaluated by the elution behaviour of hydrophobic steroids.5 The retention factors of steroids on these reported thermoresponsive copolymers remained almost constant with variation of temperatures around the LCST.9 These results indicated that a change in hydrophobic property of these grafted surfaces was not significantly affected by temperature. Besides, these reported copolymers showed higher phase transition temperature,9c,d which was not suitable for the separation of biomolecules since the relatively high temperature was required to modulate the property. For a thermally switchable surface to be usable for the enrichment applications, enhanced thermosensitivity and selective capture properties are of critical importance.
Herein, we prepared poly(NIPAAm-co-DEAEMA-co-tBAAm), a newly modified thermoresponsive chromatographic material. Grafting conformations were optimized to enhance thermosensitivity, which was examined by the elution of hydrophobic steroids. The selective capture and release properties modulated by temperature were investigated with adenosine nucleotides and proteins. Finally, the material was employed for sample preparation of human serum for proteomic analysis.
To obtain dilute copolymer chains grafted on hydrophilic surfaces, a competitive reaction of acetyl chloride with amino group was employed to reduce the amounts of atom transfer radical polymerization (ATRP)-initiating site (Scheme 1). The detailed preparation procedures are shown in ESI.† The immobilized amine group reacted with the acetyl chloride to generate amide, besides acyl bromide. Thus, ATRP initiating-site (bromide) was reduced. A thermally switchable material to be usable for enrichment via a temperature-controlled capture and release process, should have an ability for fast changes in both chain collapse and interface properties that enable rapid release on command under temperature stimuli. The rate of polymer phase transition denotes the extent of its thermosensitivity.5a The PNIPAAm chain collapse of densely packed brushes was found to occur over a broad range of temperatures.10 As a result, such brush structures of PNIPAAm could not perform critical phase transitions. By contrast, dilute polymer chains might retain a more mobile nature and hence respond more rapidly to temperature changes compared to dense brush conformation. Besides, it was also reported that proteins could readily adsorb to more dilute PNIPAAm chains, which allowed proteins to penetrate the layer and interact more extensively with the chains.11 Therefore, in this study we redesigned the grafting protocol to obtain dilute copolymer chains on the silica surface and determined whether they better satisfy the above ability.
 | ||
| Scheme 1 Schematic preparation of poly (NIPAAm-co-DEAEMA-co-tBAAm) grafted silica via surface-initiated atom transfer radical polymerization (SI-ATRP). APTES, 3-aminopropyltriethoxysilane. | ||
The copolymers have been successfully grafted onto silica surfaces, as confirmed by thermogravimetric analysis (Fig. S1†) and elemental analyses. The amount of grafted copolymer on silica surfaces per unit area was estimated to be 3.04 mg m−2. Gel permeation chromatography (GPC) analysis revealed a number-average molecular weight, Mn of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983 (Table S1, and GPC chart of the cleaved copolymer is shown in Fig. S2†). The grafting density of copolymer on silica surfaces was calculated to be 0.045 chains per nm2. In addition to fast phase transition, a competence for substantial surface property changes that facilitate the capture at one temperature and release at another temperature is indispensable. A relatively high-molecular-weight copolymer chain (Mn, 40
983 (Table S1, and GPC chart of the cleaved copolymer is shown in Fig. S2†). The grafting density of copolymer on silica surfaces was calculated to be 0.045 chains per nm2. In addition to fast phase transition, a competence for substantial surface property changes that facilitate the capture at one temperature and release at another temperature is indispensable. A relatively high-molecular-weight copolymer chain (Mn, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983) was obtained to meet this requirement.
983) was obtained to meet this requirement.
Because acetyl chloride was used for grafting it reduced the amount of bromo-groups that functioned as immobilized ATRP-initiating sites on silica surfaces resulting in dilute polymer formation, while also generating a hydrophilic grafting surface with excellent temperature sensitivity. The relatively dilute copolymer chain with a relatively high-molecular-weight copolymer chain on the hydrophilic grafting surface, which might be useful for imparting significant thermosensitivity and substantial surface property changes. A previous report showed that dilute PNIPAAm on hydrophilic grafting interface can exhibit large hydrophilicity/hydrophobicity changes at temperatures below/above the LCST.12 However, the hydrophobic interaction between proteins and PNIPAAm was so weak that PNIPAAm-grafted surfaces were unsuitable for protein adsorption.9d The interaction of copolymer and protein was strengthened by the introduction of cationic groups to the copolymer.9d However, the dense conformation of grafted copolymer chains, prepared through SI-ATRP, resulted in insufficient changes in hydrophobic properties below/above the LCST,9c,d which hampered their further application. Therefore, it was of crucial importance to optimize the architecture of the grafted copolymer and grafting surface polarity for a switchable application.
The cleaved polymer was demonstrated to have critical temperature dependent property changes, since the alteration of hydration/dehydration of grafted copolymer occurred over a relatively narrow temperature range of 6 °C (Fig. 1). LCST values at pH 7.0 and pH 7.3 were 37.4 °C and 35.2 °C, respectively. LCST values increased with a decrease in pH of the phosphate buffer, because protonation of amino groups in the copolymer was strengthened at lower pH.13 The LCST of poly(NIPAAm-co-DEAEMA-co-tBAAm) is significantly lower than those of poly(NIPAAm-co-DMAPAAm-co-tBAAm)9d and poly(NIPAAm-co-DMAEMA)9c because our DEAEMA contains more hydrophobic ethyl group in its side chains. Relative low phase transition is advantageous for the separation of biomolecules. To further examine temperature-driven changes in hydrophilic/hydrophobic properties of the grafted copolymer, we investigated the elution behaviour of hydrophobic steroids. Improved resolution, along with a retarded elution, was observed with increasing temperature (Fig. S4a†). The retention time was remarkably prolonged with increasing temperature (Fig. S4b†). These findings demonstrated that surfaces of the stationary phase underwent significant hydrophilic/hydrophobic alterations in response to thermal stimuli. Fig. S3c† shows the Van't Hoff plots of these steroids. Apparent inflection points were observed around 37.4 °C, the LCST of the copolymer, suggesting large hydration/dehydration changes with temperature across the transition temperature. Therefore, the grafted copolymer of NIPAAm with DEAEMA exhibited significant temperature-sensitivity, which could be attributed to the optimized grafting conformation.
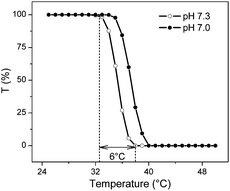 | ||
| Fig. 1 Temperature dependence of optical transmittance of 5 mg mL−1 poly(NIPAAm-co-DEAEMA-co-tBAAm) polymers suspended in 10 mM phosphate buffer. The polymers were cleaved from silica surfaces. | ||
For an excellent thermoresponsive chromatographic material to be usable for the enrichment of adenosine nucleotides such as adenosine triphosphate (ATP) via a capture and release process, ATP can be held on the material as strongly as possible at one temperature, and can be eluted as fast as possible at another temperature. In previous studies,9b,c ATP could be strongly loaded on the different copolymers at 10 °C, but the elution at 50 °C also required relatively long time (over 30 min). Although fast elution of ATP at 50 °C was obtained in the reports,9a,d no strong retention was also observed at 10 °C (less than 30 min). Therefore, those reported copolymers could only be used to separate, but not to enrich adenosine nucleotides. Here we tested whether our material was suitable for enrichment of adenosine nucleotides. As shown in Fig. 2a, an apparent increase in retention time was observed with decreasing temperatures, in particular for adenosine triphosphate (ATP). At 50 °C, all three adenosine nucleotides were rapidly eluted from the column within 5 min as a single peak. In contrast, at 25 °C two distinct peaks consisting of adenosine monophosphate (AMP) and adenosine diphosphate (ADP) were resolved, although no elution of ATP was obtained within 130 min. However, after a reverse thermal stimulation to 50 °C, a rapid release of captured ATP was observed at 100 min, which can be mostly attributed to a fast and substantial decrease in the charge density of the grafted copolymer at 50 °C, above its LCST. The success for selective capture of ATP can be explained by a presumably higher level of protonation of DEAEMA in the copolymer, because of increased electrostatic interaction with the higher numbers of phosphate units in adenosine triphosphate (ATP > ADP > AMP). The rapid release depended on the large decrease in the electrostatic interaction with increasing temperatures. The simultaneous capture of both ADP and ATP, and their selective release, were attained through temperature control using a lower pH of 7.0 (Fig. 2b). The grafted silica is pH sensitive, and a slight decrease in pH resulted in enhanced retention of ADP. At 20 °C, both ADP and ATP were captured, while adenosine and AMP were eluted rapidly with a baseline separation. This pH-dependent retention can be explained with strengthened protonation of amino groups in the copolymer. This explanation was also supported by the observation that LCST values increased with a decrease in the pH of the phosphate buffer. ADP and ATP could be released separately with a stepwise temperature increase from 20 to 40 °C and 40 to 50 °C, respectively. The grafted silica was successfully applied towards sample enrichment. A 2.62-fold enrichment of ADP and 2.80-fold enrichment of ATP were obtained after three repetitions of injections via the temperature-controlled capture and release method (Fig. S5†).
In the described method, temperature seemed to act as a switchable signal that can be turned “on/off” for the capture and release of target molecules. NIPAAm moieties were crucial for the release of adenosine nucleotides, whereas the capture depended on the DEMEMA units. As discussed above, the capture and rapid release abilities relied on the substantial and fast change in the charge properties of grafted copolymers. The simultaneous separation and concentration of adenosine nucleotides could be achieved by simply modulating the charge property with temperature stimuli. This convenient and fast technology provides great practical advantages over conventional approaches by eliminating the time-consuming need for buffer-changing steps, or the use of organic solvents.
To investigate the selective capture properties of proteins on poly(NIPAAm-co-DEAEMA-co-tBAAm) column, a sample with 8 proteins of different molecular weights and isoelectric points was selected as a model. Dramatic temperature-dependent changes in retention times were observed for bovine serum albumin (BSA) and human serum albumin (HSA); however, the other 6 proteins including cytochrome c, lysozyme, ribonuclease A (Rnase A), myoglobin, carbonic anhydrase, and α-glucosidase showed no retention at temperatures ranging from 10 to 40 °C (Fig. S6†). At 10 °C, both BSA and HSA were not retarded, whereas at 40 °C they were completely captured by the column within 60 min. Interestingly, the captured proteins were rapidly released upon reducing the temperature to 10 °C. This illustrates that selective capture and release of proteins could be achieved by modulating the surface property of the chromatographic material, simply by shifting the column temperature between 40 °C and 10 °C. At 40 °C, the copolymer exhibited hydrophobic property and the chains collapsed, the target proteins were captured due to strong interactions between proteins and copolymers (Scheme 2). As the temperatures instantaneously changed from 40 °C to 10 °C, the copolymer chains became hydrophilic and expanded. The changes in conformation and hydrophobicity weakened the interaction of the captured proteins with stationary phase. Thus, the captured target proteins were rapidly released. The adsorption capacity of the prepared column at 40 °C was 2.83 mg of HSA per column by frontal chromatography. In a previous report, HSA was also found to adsorb to a different poly(NIPAAm-co-DMAPAAm-co-tBAAm) column within 8 min at 40 °C until the temperature was lowered to 10 °C.9d Notably, in our study the captured proteins HSA and BSA could be retarded for a long duration (over 60 min) on the column; therefore, this process has a great potential for the enrichment of target proteins.
 | ||
| Scheme 2 Schematic illustration of the thermally modulated capture and release of target proteins on the poly(NIPAAm-co-DEAEMA-co-tBAAm) grafted silica surface. | ||
The enrichment efficiency of HSA on poly(NIPAAm-co-DEAEMA-co-tBAAm) grafted silica column was further examined. The grafted copolymers selectively absorbed HSA from a mixture of HSA, lysozyme, and myoglobin at 40 °C, and then rapidly released it at 10 °C (Fig. 3a). When the mixture of the three proteins was injected into the column for five times at 40 °C, with equal amounts every time, both lysozyme and myoglobin were quickly eluted after injection while HSA was retained in the column. When the temperature was lowered to 10 °C, the captured HSA was rapidly released with an increased peak area (Fig. 3b). The enrichment efficiency was up to 87.2%. The results thus indicated that it was possible to manipulate the prepared column effectively for enrichment of proteins via a thermally controlled capture and release mechanism.
The human serum is a complex bio-fluid. Removal of abundant proteins from complex biological samples has been demonstrated to vastly increase the number of individual proteins that can be identified in the mix.1a HSA is one of the most abundant proteins in human serum. The poly(NIPAAm-co-DEAEMA-co-tBAAm) grafted silica column was tentatively employed for proteomic analysis. By means of temperature-modulated capture and release of grafted silica, the crude human serum was divided into two fractions of captured and uncaptured proteins. The protein samples were then digested and analyzed by LC/MS/MS for identification. As shown in Fig. 4, 174 and 276 proteins could be identified from the captured and uncaptured fractions, respectively. Thus, a total of 433 proteins were identified from the human serum sample with the capture/release pretreatment, while only 216 proteins were identified without the capture/release pretreatment. Thus, the thermally-modulated capture and release strategy doubled the number of identified proteins. It was also observed that the pretreatment of crude serum by passage through a prepared thermoresponsive column not only increased the total number of identified proteins, but also greatly enhanced the score and coverage of most identified proteins (Table S2†). The capture and release of the target proteins using the prepared column can be simply done by changing the column temperature without the conventional need to change the composition of the mobile phase that is not compatible with MS analysis. These results reveal the remarkable potential of the prepared thermoresponsive material in the separation and enrichment of proteins from complex biological matrices.
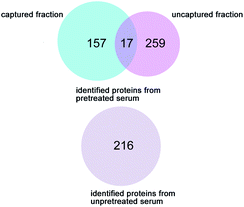 | ||
| Fig. 4 Comparison of the number of individual human serum proteins identified in samples that were pretreated with poly(NIPAAm-co-DEAEMA-co-tBAAm) grafted silica columns and in untreated samples. | ||
In conclusion, we prepared a thermosensitive chromatographic material, poly(NIPAAm-co-DEAEMA-co-tBAAm)-grafted silica featuring a switchable property. The grafting configuration was optimized to achieve apparent temperature-dependent retention changes for steroids, adenosine nucleotides, and proteins. A selective enrichment and separation via a thermally-modulated capture and release method was achieved for adenosine nucleotides and proteins such as HSA and BSA. In addition, the column was tested for a proteomics analysis of crude human serum, resulting in a significant increase in the number of identified proteins. This indicates that the prepared thermoresponsive material may have great potential as a selective chromatographic material for the separation and enrichment of proteins from different complex biological matrices.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 program, 2012CB91063) and the National Eleventh-Five Year Research Program of China (2009bak59b03). We thank Dr Gaofei Hu for the assistance of 1HNMR measurement and also appreciate to Prof. Michael R. Schläppi for English editing.Notes and references
- (a) K. K. Unger, R. Ditz, E. Machtejevas and R. Skudas, Angew. Chem., Int. Ed., 2010, 49, 2300 CrossRef CAS PubMed; (b) A. Jungbauer, J. Chromatogr. A, 2005, 1065, 3 CrossRef CAS PubMed.
- C. Hou, J. Ma, D. Tao, Y. Shan, Z. Liang, L. Zhang and Y. Zhang, J. Proteome Res., 2010, 9, 4093 CrossRef CAS PubMed.
- (a) Y. Xu, Z. Wu, L. Zhang, H. Lu, P. Yang, P. A. Webley and D. Zhao, Anal. Chem., 2009, 81, 503 CrossRef CAS PubMed; (b) Y. Liu, L. Ren and Z. Liu, Chem. Commun., 2011, 47, 5067 RSC; (c) L. Liang and Z. Liu, Chem. Commun., 2011, 47, 2255 RSC.
- S. Andrecht, J. Anders, R. Hendriks, E. Machtejevas and K. K. Unger, Laborwelt, 2004, 5/2, 4 Search PubMed.
- (a) P. Maharjan, B. W. Woonton, L. E. Bennett, G. W. Smithers, K. DeSilva and M. T. W. Hearn, Innovative Food Sci. Emerging Technol., 2008, 9, 232 CrossRef CAS; (b) H. Kanazawa and T. Okano, J. Chromatogr. A, 2011, 1218, 8738 CrossRef CAS PubMed.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Part A: Pure Appl. Chem., 1968, 2, 1441 CrossRef CAS.
- (a) H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi, Y. Sakurai and T. Okano, Anal. Chem., 1996, 68, 100 CrossRef CAS PubMed; (b) H. Kanazawa, M. Nishikawa, A. Mizutani, C. Sakamoto, Y. Morita-Murase, Y. Nagata, A. Kikuchi and T. Okano, J. Chromatogr. A, 2008, 191, 157 CrossRef PubMed; (c) A. Mizutani, K. Nagase, A. Kikuchi, H. Kanazawa, Y. Akiyama, J. Kobayashi, M. Annaka and T. Okano, J. Chromatogr. A, 2010, 1217, 5978 CrossRef CAS PubMed; (d) R. Y. Zhang, G. L. Yang, P. Y. Xin and L. Qi, J. Chromatogr. A, 2009, 1216, 2404 CrossRef CAS PubMed; (e) M. Q. Liu, H. Y. Liu, Y. K. Liu, L. G. Bai, G. L. Yang, C. L. Yang and J. Cheng, J. Chromatogr. A, 2011, 1218, 286 CrossRef CAS PubMed; (f) P. Y. Xin, Y. Shen, L. Qi, G. L. Yang and Y. Chen, Talanta, 2011, 85, 1180 CrossRef CAS PubMed.
- (a) R. J. Dai, X. Zhang, N. Hu, Y. Liu and Y. L. Deng, Electrophoresis, 2009, 30, 616 CrossRef CAS PubMed; (b) R. J. Dai, L. Chen, Z. J. Liu, H. H. Wang, D. Y. Hu and Y. L. Deng, J. Appl. Polym. Sci., 2011, 121, 2233 CrossRef CAS; (c) Z. J. Liu, Y. L. Liang, F. F. Geng, C. Ge, K. Ullah, F. Lv, R. J. Dai, Y. K. Zhang and Y. L. Deng, J. Sep. Sci., 2012, 35, 2069 CrossRef CAS PubMed; (d) Z. J. Liu, Y. L. Liang, F. F. Geng, F. Lv, R. J. Dai, Y. K. Zhang and Y. L. Deng, Front Mater. Sci. China, 2012, 6, 60 CrossRef; (e) Z. J. Liu, K. Ullah, L. P. Su, F. Lv, Y. L. Deng, R. J. Dai, Y. J. Li and Y. K. Zhang, J. Mater. Chem., 2012, 22, 18753 RSC; (f) Z. J. Liu, F. F. Geng, X. F. Ha, Y. S. Feng, B. Q. Che, K. C. Wu, Y. J. Li, R. J. Dai, Y. K. Zhang and Y. L. Deng, Chromatographia, 2013, 76, 201 CrossRef CAS.
- (a) E. Ayano, C. Sakamoto, H. Kanazawa, A. Kikuchi and T. Okano, Anal. Sci., 2006, 22, 539 CrossRef CAS PubMed; (b) A. Kikuchi, J. Kobayashi, T. Okano, T. Iwasa and K. Sakai, J. Bioact. Compat. Polym., 2007, 22, 575 CrossRef CAS; (c) K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Biomacromolecules, 2008, 9, 1340 CrossRef CAS PubMed; (d) K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Biomaterials, 2011, 32, 619 CrossRef CAS PubMed.
- S. Balamurugan, S. Mendez, S. S. Balamurugan, M. J. O'Brien II and G. P. López, Langmuir, 2003, 19, 2545 CrossRef CAS.
- C. Xue, N. Yonet-Tanyeri, N. Brouette, M. Sferrazza, P. V. Braun and D. E. Leckband, Langmuir, 2011, 27, 8810 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, M. Annaka, H. Kanazawa and T. Okano, Langmuir, 2008, 24, 10981 CrossRef CAS PubMed.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Macromolecules, 1993, 26, 2496 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra41454j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |


