A dendrimer matrix for performance enhancement of evanescent wave absorption-based fiber-optic biosensors†
Jitendra Satijaa,
Bhuvaneshwari Karunakarana and
Soumyo Mukherji*abc
aDepartment of Biosciences and Bioengineering, IIT Bombay, Mumbai 400 076, India. E-mail: mukherji@iitb.ac.in; Fax: +91 22 2572 3480; Tel: +91 22 2576 7767
bCentre for Research in Nanotechnology and Science, IIT Bombay, Mumbai 400 076, India
cCentre of Excellence for Nanoelectronics, IIT Bombay, Mumbai 400 076, India
First published on 12th February 2014
Abstract
Dendrimers are monodispersed, hyperbranched macromolecules which have a globular shape and high pendant group density. Due to these unique properties, dendrimers have been used for catalytic, biochemical, pharmaceutical, biomedical and energy transfer applications. This study demonstrates the potential of a dendrimeric biointerface for the immobilization of bioreceptors, and the overall improvement of the performance of evanescent wave absorbance-based fiber-optic biosensors. Amine functionalized sensor substrates obtained by fourth generation poly(amidoamine) dendrimer immobilization were compared with substrates obtained by a conventional silanization technique that used aminopropyl triethoxysilane. A fluorescein isothiocyanate-based amine assay and X-ray photoelectron spectroscopy showed that the dendrimer matrix had 2.4 times the number of amine groups of the silanized surface. Further, atomic force microscopy-based topographic analysis revealed that the dendrimer matrix caused a 1.3-fold increase in surface area compared to the silane matrix, which caused a negligible change. In addition to a two-fold improvement in antibody loading compared to conventionally functionalized probes, an eight-fold increase in the sensor response for critically important lower concentrations of analyte was observed with dendrimer coated U-bent fiber-optic probes. Our detailed experimental studies demonstrated the potential of the dendrimer for preparing a biosensing platform with high protein loading, amplified sensor response and low nonspecific protein binding.
Introduction
Evanescent wave absorption-based fiber-optic sensors are of great interest for diagnostic applications because of their (i) immunity to interference from molecules in the bulk of the sample, (ii) high sensor performance, (iii) simplicity of design and (iv) ease of fabrication. These sensors have been successfully utilized for the detection of glucose,1 DNA,2 proteins3 and microorganisms.4 The performance of these sensors depends upon various factors, e.g. the bioreceptors’ surface density and spatial distribution, the extinction coefficient of the analyte, the surface area for interaction of the analyte with the waveguide, the sensor geometry, the wavelength of the propagating light and the refractive index of the waveguide and surrounding medium, etc.5,6There has been tremendous progress in improving the efficiency of optical fiber sensors by employing (i) a labeled approach using fluorophores,7 (ii) a label-free approach using metal nanoparticles or nanocomposites,8 (iii) a sandwich-type assay,9, and so on. Modulation of the optical parameters (e.g. fiber core diameter, probe length, light source, etc.) and creation of newer types of geometrical designs also led to a significant improvement in sensor performance.5 In particular, tapered and U-shaped optical fiber probes have shown great potential for enhanced sensitivity.10
Efforts have been made to improve the linker chemistry of the optical fiber sensor surface, which plays an essential and vital part in any sensor. Most scientists have focused their attention on two-dimensional linkers, e.g. silanes, poly-L-lysine, polyaniline, etc. or three-dimensional gels for sensor matrix design. However, several challenges exist, such as issues with the reproducibility of the matrix, inadequate physicochemical stability, restricted bioreceptor loading efficiency, loss of functionality of the bioreceptors at these sensor surfaces, and limited analyte interaction.11–13 To circumvent these problems, it is essential to explore either a new bioconjugation protocol, or advanced linker molecules.
During the last decade, research in the field of diagnostics has included the exploration of supramolecular dendrimeric architectures for sensor matrix fabrication.14,15 Dendrimers are monodispersed macromolecules with regular and highly branched three-dimensional structures. These nanoscale architectures have dense multidentate homo- or heterogeneous terminal functional groups. These are promising moieties for the attachment of biomolecules such as antibodies, enzymes, DNA, etc.16 Most of the properties of dendrimers are governed by these peripheral functional groups, which depend upon the generation and type of the dendrimer. Typically, the number of surface functional groups increases as a function of dendrimer generation, e.g. a first generation (1G) poly(amidoamine) (PAMAM) dendrimer has 8 surface amino groups while a 4G dendrimer possesses 64 amine groups.17
Among the different generations of dendrimer, biomedical researchers prefer to use those of fourth and/or fifth generation, because these generations exhibit the full range of dendritic properties, i.e. globular shape, structural uniformity, dendrimeric crevices, high physicochemical stability, compact structure, etc.18,19 In contrast, the properties of lower generation dendrimers are highly similar to linear polymers or linker molecules; therefore these generations have limited applications in biomedical domains.20 On the other hand, higher generation dendrimers (>6G) have shown weaker receptor–analyte interactions due to steric crowding on the dendrimer surface.21 Thus, fourth or fifth generation dendrimers are preferred for analytical, diagnostic and nanomedical fields.22–24 Trévisiol et al.25 demonstrated the high binding capacity of oligonucleotides on dendrimer modified slides (dendrislides) compared to commercially available aldehyde slides. Ajikumar et al.26 reported high protein loading and less nonspecific binding on 4.5G poly(amidoamine) dendrimers compared to linear linkers and lower generation dendrimers. Similarly, some other structural and compositional properties of dendrimers have been shown to significantly improve the performance of electrochemical,14 fluorescence,15 surface enhanced Raman scattering,27 gravimetric,28 surface plasmon resonance,29 and impedimetry30 based sensors. However, the application of dendrimers as an interfacing layer for optical fiber biosensors has not been established. Recently, our group investigated the potential of dendrimers in enhancing the response of a localized surface plasmon resonance-based optical fiber sensor.31 The dendrimer matrix enhanced the sensor performance by 1.4 times compared to a silane matrix.
The present study aims to apply such dendrimer matrices to evanescent wave absorption-based optical fiber biosensors. It can be logically expected that this will enhance the sensor response, but to our knowledge there is no study that has performed such experiments. Further, a detailed characterization of dendrimer matrices on optical fibers is conspicuously absent from published literature. In this study, dendrimer and silane films were deposited on U-shaped fiber-optic probes and characterized with regards to: (i) surface area and surface topography, (ii) amine group quantification, (iii) bioreceptor loading efficiency, (iv) immunodetection, and (v) degree of nonspecific binding.
Materials and methods
Materials
Optical fibers with a core diameter of 200 μm (numerical aperture = 0.37) were procured from CeramOptec®. 1,1′-Carbonyldiimidazole (CDI), 3-aminopropyl triethoxysilane (APTES), amine-terminated fourth generation (4G) PAMAM dendrimers, fluorescein isothiocyanate (FITC) on Celite, phosphate buffer saline (PBS, 10 mM and pH 7.4), and fluorescein isothiocyanate-tagged human immunoglobulin G (FITC-HIgG) antibodies were purchased from Sigma-Aldrich. Bovine serum albumin (BSA), human IgG and FITC-tagged goat antihuman IgG (FITC-GaHIgG) antibodies were obtained from Bangalore Genei, India. 1,4-Dioxane was procured from Merck, India. Acetic acid and glutaraldehyde were obtained from SD Fine Chemicals, India. All other chemicals used were of analytical grade and used without further modification. Deionized (DI) water was used for the cleaning and preparation of solutions.Fabrication and characterization of the U-shaped probes
U-shaped optical fiber probes were fabricated and characterized as described previously by our group.10 In brief, a decladded portion (2 cm) in the middle of a 40 cm long piece of optical fiber was bent into a ‘U’ shape with the help of a butane candle flame, to obtain probes with a bend diameter of ∼1.5 mm. The bent portion was observed under an optical microscope to measure the bending diameter and uniformity in the bent region. Probes with a bend diameter of 1.4–1.6 mm were accepted and the rest were discarded. Probes in which the bending resulted in torsional stress and an out-of-plane bend region were also rejected.Functionalization of the optical fiber probes
Prior to surface modification, the optical fiber probes were cleaned as explained previously.32 First the probes were dipped in methanol–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 60 min, and this was followed by thorough rinsing with DI water. Then the probes were incubated in conc. H2SO4 for 60 min, which was followed by exhaustive rinsing with DI water. These cleaned probes were treated with sulfochromic acid for 10 min, to generate silanol sites, and were then washed thoroughly with DI water and dried under nitrogen.
1 v/v) for 60 min, and this was followed by thorough rinsing with DI water. Then the probes were incubated in conc. H2SO4 for 60 min, which was followed by exhaustive rinsing with DI water. These cleaned probes were treated with sulfochromic acid for 10 min, to generate silanol sites, and were then washed thoroughly with DI water and dried under nitrogen.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v mixture of ethanol and acetic acid) for 10 min. Acetic acid was added to maintain the orientation of the amine groups of APTES away from the surface.33 After that, the probes were rinsed thrice with ethanol to remove any loosely bound silane. This was followed by condensation of the bound silane, by heating the treated probes at 115 °C for at least 90 min.
2 v/v mixture of ethanol and acetic acid) for 10 min. Acetic acid was added to maintain the orientation of the amine groups of APTES away from the surface.33 After that, the probes were rinsed thrice with ethanol to remove any loosely bound silane. This was followed by condensation of the bound silane, by heating the treated probes at 115 °C for at least 90 min.Characterization of the functionalized matrices
Both types of sensor matrix were characterized qualitatively and quantitatively for the presence of amine groups, using a FITC-based amine assay and an X-ray photoelectron spectroscopy (XPS) study. FITC is a fluorescent dye that is well-known for its affinity towards primary amine groups.36 This property was used to confirm the binding of silane and dendrimers on the optical fiber. Silane- and dendrimer-functionalized optical fiber probes were immersed in FITC solution (50 μM, prepared in 25 mM borate buffer, pH 8.3) for 30 min then washed thrice with borate buffer. The FITC treated probes were observed under a fluorescence optical microscope (ZEISS Axioskope-2 MAT). A FITC binding study was also carried out on control samples, i.e. bare and CDI coated optical fiber probes. Furthermore, the amine groups on the silane and dendrimer matrices were quantified, as explained previously.37,38 The immobilized FITC molecules were eluted by incubating the fiber probes in tri-sodium phosphate buffer (100 mM, pH 12.21) for 12 h. The eluted samples were analysed to find the FITC concentration, using a spectrofluorophotometer (RF-5301PC, Shimadzu Scientific Instruments). The FITC concentration was calculated against the calibration curve of FITC in tri-sodium phosphate buffer solution, with a concentration range of 100–1000 nM (Fig. S1 in the ESI†).X-ray photoelectron spectroscopy measurements were performed using a Thermo VG Scientific Multilab 2000 system with monochromatic Al Kα radiation (energy = 1486.6 eV) and a hemispherical electron energy analyzer with a nine-channel detector. The system was operated at a main chamber base pressure of 4 × 10−10 mbar. A survey scan (1000–0 eV) was carried out with a step size of 1 eV, and an elemental scan for C, N, and O was carried out with a step size of 0.05 eV. The peaks were fitted with XPS Peak 4.1 software.
Since the surface roughness of the sensor matrix modulates the surface area for interactions and physical adsorption and/or non-specific binding,39 a three dimensional topographic analysis of both types of matrix was done using atomic force microscopy (AFM). As the AFM scanning was difficult to perform on the cylindrical optical fiber surface, the sensor matrices were fabricated on a flat glass surface (7 × 7 mm). A Digital Instrument Nanoscope III instrument was used in tapping mode.
Evaluation of the antibody immobilization efficiency
To test the antibody loading efficiency of the sensor surface, FITC-HIgG antibodies were used. Before antibody binding, both dendrimer- and silane-coated probes were treated with an aqueous solution of glutaraldehyde (1% v/v) for 60 min followed by washing thrice with DI water. Thereafter, these probes were incubated in FITC-HIgG antibody solution (100 μg mL−1 prepared in PBS buffer, pH 7.4), and the binding of the antibodies was studied in real-time using the optical set-up (Fig. 1). The absorbance due to binding of the FITC-HIgG antibodies on the glutaraldehyde-activated matrices was recorded, using a portable fiber-optic spectrophotometer (USB 4000, Ocean Optics®, USA). The absorbance measurement for the buffer solution was taken as the background reference in these experiments. After ∼2.5 h of incubation, the probes were washed thoroughly with PBS to remove any loosely adsorbed antibodies.Evaluation of the immunodetection capacity
To assess the performance of the immunoassay on dendrimer and silane matrices, FITC-GaHIgG antigen were selected as an analyte while the untagged HIgG antibodies were used as bioreceptors. HIgG antibodies (100 μg mL−1 prepared in PBS buffer, pH 7.4) were immobilized on both types of matrix after glutaraldehyde activation. HIgG antibody-bound probes were washed thrice with PBS solution followed by BSA treatment (concentration = 2 mg mL−1 in PBS) for 30 min. BSA helps to reduce the amount of non-specific binding that occurs, by covering the free aldehyde functional groups on the sensor surface.40 Thereafter, the probes were washed thrice with PBS solution and used for immunodetection. The immunoassay experiments were performed in real-time on the optical set-up (Fig. 1) and the absorbance change due to the binding of the FITC-GaHIgG biomolecules to the HIgG coated fiber probes was recorded. Both types of matrix were tested with different concentrations of FITC-GaHIgG antigen, ranging between 0.1 μg mL−1 to 50 μg mL−1. The absorbance measurement for the buffer solution was taken as the background reference in these experiments.Results and discussion
Characterization of the functionalized matrices
Fig. 2 depicts the fluorescence images of various functionalized optical fiber probes after FITC treatment. Both silane- and dendrimer-coated probes showed a bright fluorescence signal, indicating substantial binding of FITC. This was possibly due to the availability of the amine groups. On the other hand, the control samples (bare and CDI coated probes) displayed a very faint signal, suggesting very poor/no binding of FITC due to the absence of amine groups. Further, a FITC-based amine assay was performed to determine the density of the amine groups. In the silane matrix, the measured amine group density was found to be 4.3 ± 0.5 amine groups per nm2, while the dendrimer matrix had 10.3 ± 0.4 amine groups per nm2. The measured amine group density on the silane and dendrimer matrices is in good agreement with previous investigations.41,42 The 2.4-fold higher amine group density in the dendrimer matrix was due to the multivalent terminal amine groups of dendrimers. | ||
| Fig. 2 Fluorescence microscopic images of various functionalized fiber-optic probes after treatment with FITC. The scale bars in all images represent 100 μm. | ||
Fig. 3 shows the XPS spectra of silane- and dendrimer-immobilized glass substrates. A survey scan of both types of matrix showed the presence of C (1s), N (1s) and O (1s), at 284.6, 399.5, 532.7 eV, respectively. In addition, Si (2p) and Si (2s) peaks were observed in both types of modified substrate, at 103 eV and 153 eV, respectively. This was due to the presence of silica in the glass substrate and the silane molecules. Additionally, a peak near 500 eV corresponded to an auger transition of sodium (Na-KLL).43 The presence of an N (1s) peak in both types of matrix confirmed the binding of the silane and the dendrimers on the glass substrate.
Though the photoelectron counts indicating the presence of an N (1s) peak in the survey scans of the silanized substrates were very low, due to the monolayer of silane, a high resolution XPS showed a well defined N (1s) peak (Fig. 3a inset image). The peak fitted N (1s) region showed two peaks at 400.7 eV and 399.1 eV, corresponding to the protonated and unprotonated primary amine groups of APTES. This is consistent with the literature.44,45 The surface N content of the silanized sample was found to be 2.8 atom%, which further suggests the formation of a silane monolayer.46 On the other hand, the high resolution N (1s) region of the dendrimer-immobilized surface (Fig. 3b inset image) exhibited a short tailing on the low binding energy side of the main energy peak, as observed previously by other groups.47 As a result, this peak was deconvoluted into two peaks at 400.1 eV and 399.2 eV, corresponding to the amide and amine groups, respectively. The energy difference of 1.1 eV between the amine and amide peak is in good agreement with previous findings.48 The Ndendrimer/Nsilane ratio (for NH2 peak only) was found to be 2.3, which suggests that the number of amine groups on the dendrimer-functionalized surfaces is more than double the number on the silanized surfaces. This is in agreement with the results obtained from the FITC-based amine assay.
Fig. 4 shows the 3D topographic AFM images of different sensor matrices fabricated on a flat glass surface. Bare glass and CDI coated glass substrates revealed a smooth and almost atomically flat surface, with root mean square (rms) roughnesses of 0.18 ± 0.03 nm and 0.17 ± 0.04 nm, respectively. Furthermore, silanization of the bare glass increased its rms roughness to 0.23 ± 0.05 nm. This small change may be due to the heterogeneous spatial distribution of the silane molecules. This also suggests the presence of a 2D silane matrix. In contrast, the dendrimer matrix revealed grooved and dome shaped features throughout the surface, with an average rms roughness of 0.76 ± 0.06 nm. The heights of these features were in the range of 2–5 nm, suggesting flattening of the dendrimer molecules on the sensor surface. This morphological change of the dendrimers is attributed to clustering and collapse of the globular shaped molecules. These results are consistent with previous AFM studies of dendrimer films, showing the dendrimer molecules in highly flattened configurations.49
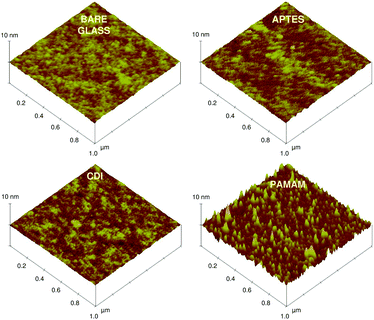 | ||
| Fig. 4 3D topographic AFM height images of plain glass, 3-APTES coated glass, CDI coated glass and PAMAM (4G) dendrimer coated glass substrates (pre-activated with CDI). | ||
Furthermore, topographic analysis was carried out to find the height profiles and surface area measurements of all the matrices, using a program written in MATLAB 6.5 software (Sections S1 and S2 in the ESI† for height profile and surface area measurements). No significant changes in average height were observed after silane or CDI coating on the bare glass (Table 1). In contrast, the dendrimer matrix revealed the presence of ellipsoid or dome shaped patterns with a two-fold increase in the average height. The ellipsoid shape of the dendrimers in the sensor matrix is more beneficial than their spherical shape (in solution), as it could help cause a greater amount of polyvalent interactions due to its complementary shape.20 Because of this, the dendrimer matrix increased the surface area 1.3-fold compared to the silane matrix, which showed a negligible change compared to the glass surface. This increase in surface area by the dendrimers is helpful for any biosensor design, as it may improve the bioreceptors’ immobilization efficiency.
| Matrix type | RMS roughness (nm) | Average height (nm) | Surface area for 1 um × 1 um of sensor surface (μm2) |
|---|---|---|---|
| a For each matrix type n = 3 (i.e. AFM images were taken three times for each type of matrix). As the average height variations in the bare, CDI and APTES coated surfaces were negligible, the surface area of these three matrices was assumed to be nearly the same (i.e. ∼1 μm2 for 1 um × 1 um of sensor surface). For surface area calculations on the PAMAM dendrimer matrix, ellipsoid features were assumed to exist on the sensor surface (see ESI for more detail)†. | |||
| Bare | 0.18 ± 0.03 | 1.83 ± 0.18 | ∼1.0 |
| CDI | 0.17 ± 0.04 | 1.83 ± 0.18 | ∼1.0 |
| APTES | 0.23 ± 0.05 | 1.85 ± 0.26 | ∼1.0 |
| PAMAM | 0.76 ± 0.06 | 3.65 ± 0.43 | ∼1.3 |
Antibody loading efficiency
The antibody loading efficiency was evaluated for both the dendrimer and silane matrices, using FITC-HIgG antibodies. These show excitation maxima at 495 nm (Fig. S3 in the ESI†). Fig. 5a shows the absorbance spectra obtained from the binding of the FITC-HIgG antibodies to different types of sensor matrix. Fig. 5b depicts the real-time changes in absorbance at 495 nm due to the binding of FITC-HIgG antibodies on all types of sensor surface. Antibody loading was found to be significantly higher on the dendrimer surface compared to the silanized and control surfaces (P value < 0.001) (Fig. 5c). The control surfaces, i.e. bare and CDI coated probes, showed poor binding of antibodies due to the absence of amine and subsequently aldehyde functional groups, after glutaraldehyde activation.Comparing the dendrimer and silane matrices, it was observed that both matrices displayed an initially rapid loading of antibodies, followed by a slower binding rate and then a plateau. This suggests the dynamic equilibrium state of bioreceptor loading. The average values (n = 3) of A495 nm for silane- and dendrimer-functionalized probes were found to be 0.019 ± 0.004 and 0.042 ± 0.005, respectively. This indicates that the number of bound antibodies is more than twice as high on the dendrimer matrix as on the silane matrix. Statistical analysis (Student's t-test) suggested that the antibody loading efficiency was significantly higher on the dendrimeric platform than on the silane matrix (P value < 0.02). The higher antibody binding efficiency on the dendrimer matrix is attributed both to the greater number of amine groups and to the ellipsoid shape of the dendrimers in the sensor matrix, which provided a greater surface area for bioconjugation. Additionally, the three dimensional structure of the dendrimers might have helped in the reduction of steric hindrance of the activated terminal amine groups, making a large number of reactive groups available for antibody coupling.29 These results are in agreement with previous studies where dendrimer linkers showed a 2-fold enhancement in DNA and protein immobilization compared to conventional linear linkers.15,50 This high receptor loading efficiency of dendrimer matrices not only enhances the signal-to-noise ratio51 but is also advantageous for evanescent wave absorption based near field sensing.52
Immunodetection capacity
In order to evaluate the performance of the silane and dendrimer matrices in an immunoassay, FITC-tagged GaHIgG antibodies were used as an analyte, and they have an absorbance peak at 495 nm. For this, both dendrimer- and silane-functionalized fiber probes were first coated with HIgG antibodies and treated with BSA, and then exposed to a wide range of analyte (FITC-GaHIgG) concentrations from 0.1 to 50 μg mL−1 (prepared in 10 mM PBS buffer pH 7.4). Each type of sensor probe was firstly incubated with 0.1 μg mL−1 FITC-GaHIgG for 30 min, followed by successively higher concentrations of the analyte. The sensor response, i.e. the change in absorbance at 495 nm, was recorded at intervals of five seconds.Fig. 6a shows a comparative time-resolved immunoassay response curve obtained from the HIgG-coated dendrimer- and silane-functionalized probes. Saturated responses (after incubation of 30 min with analyte solution) of the silane- and dendrimer-immobilized probes for different concentrations of FITC-GaHIgG were plotted to obtain a dose response curve, as shown in Fig. 6b.
The time-resolved curve shown in Fig. 6a confirms that the expected interaction of the FITC-GaHIgG and the HIgG antibodies occurred on both types of matrix. This suggests that the HIgG antibodies retained their functionality after being immobilized. No significant drop in absorbance was observed after flushing the flow cell with PBS at the end of the experiment, indicating no desorption of the bound analyte from either type of matrix. It can be seen from Fig. 6a that for each analyte concentration, the response from the dendrimer matrix was at least twice that of the silane matrix. Interestingly, at low analyte concentrations (0.1 μg mL−1 and 1 μg mL−1), the average values of A495 nm for the dendrimer matrix were 0.0027 ± 0.0003 and 0.0157 ± 0.0038, which is 8 and 4.7 times higher than those of the silane matrix, respectively. Most likely, this is due to an increased amount of receptor antibodies tethered to the amino-functionalized dendrimer molecules attached to the fiber probe.51 In addition, the three dimensional dendrimer matrix might have contributed to some extent in reducing steric hindrance during the antigen binding. A two-way ANOVA test between the dendrimer and silane matrices for different analyte concentrations concluded that the dendrimer matrix improved the sensor response significantly (P value < 0.0001). The response of the dendrimer matrix was found to be better than a reported study in which a sol–gel based matrix was used in a fiber-optic biosensor for protein measurement.53
Determination of non-specific binding
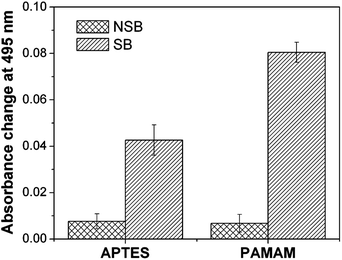
In a separate study, the degree of non-specific binding was evaluated on both the dendrimer and silane matrices. For this, rabbit IgG antibodies, which are non-specific to FITC-GaHIgG antigens, were immobilized on glutaraldehyde activated dendrimer and silane matrices. A 10 μg mL−1 concentration of FITC-GaHIgG was used for the immunoassay and the absorbance response was monitored at 495 nm. It was observed that the sensor response for non-specific binding was nearly the same for the silane (A495 = 0.008 ± 0.003) and dendrimer functionalized probes (A495 = 0.007 ± 0.004) (Fig. S4 in the ESI†). Since the specific binding response is 2.2-fold higher for dendrimer coated probes compared to silanized probes, the ratio of non-specific binding to specific binding (NSB/SB) for the dendrimer matrix (0.08) was approximately half that of the silane matrix (0.18) (Fig. 7). This indicates that the dendrimeric surface produces a higher signal-to-noise ratio than the silanized surface. The NSB can be further reduced by using hydroxyl- or carboxyl-terminated dendrimers, or poly-ethylene glycol as a NSB blocker.Conclusion
We have demonstrated the potential of dendrimer matrices over silane matrices for use in evanescent wave absorption-based fiber-optic biosensors. The globular shape and high pendent group density of the dendrimeric architecture provides an increased surface area and a greater number of amine groups. Compared to conventional APTES, a dendrimer matrix enhances the loading efficiency of bioreceptor molecules almost 2.2-fold. During an immunoassay, the dendrimer based sensors show a significantly higher response for antigen detection. The sensor response is nearly 8-fold higher in the lower concentration range. This enhanced sensor performance may be due to the inherent properties of the dendrimer molecules, which produce a 3-dimensional interaction matrix. Further, multidentate dendrimers can be exploited to develop multiplexed fiber-optic biosensors. These can target multiple biomarkers at the same time for the detection of afflictions like myocardial infarction, and may enhance the speed and efficacy of detection.Acknowledgements
The authors acknowledge the FIST (Physics)-IRCC central SPM facility and the Central Surface Analytical Facility of IIT Bombay for AFM and XPS measurements. J. Satija thanks Dr V.V.R. Sai, Nirmal Punjabi, Joseph Tharion and Vishal Anvekar for their help.Notes and references
- S. K. Srivastava, V. Arora, S. Sapra and B. D. Gupta, Plasmonics, 2012, 7, 261 CrossRef CAS.
- X. Liu and W. Tan, Anal. Chem., 1999, 71, 5054 CrossRef CAS PubMed.
- M. Lee and D. R. Walt, Anal. Biochem., 2000, 282, 142 CrossRef CAS PubMed.
- R. Bharadwaj, V. V. R. Sai, K. Thakare, A. Dhawangale, T. Kundu, S. Titus, P. K. Verma and S. Mukherji, Biosens. Bioelectron., 2011, 26, 3367 CrossRef CAS PubMed.
- A. Leung, P. M. Shankar and R. Mutharasan, Sens. Actuators, B, 2007, 125, 688 CrossRef CAS.
- V. V. R. Sai, T. Kundu, C. Deshmukh, S. Titus, P. Kumar and S. Mukherji, Sens. Actuators, B, 2010, 143, 724 CrossRef CAS.
- F. Kleinjung, F. F. Bier, A. Warsinke and F. W. Scheller, Anal. Chim. Acta, 1997, 350, 51 CrossRef CAS.
- C.-Y. Chiang, M.-L. Hsieh, K.-W. Huang, L.-K. Chau, C.-M. Chang and S.-R. Lyu, Biosens. Bioelectron., 2010, 26, 1036 CrossRef CAS PubMed.
- U. Narang, G. P. Anderson, F. S. Ligler and J. Burans, Biosens. Bioelectron., 1997, 12, 937 CrossRef CAS PubMed.
- V. V. R. Sai, T. Kundu and S. Mukherji, Biosens. Bioelectron., 2009, 24, 2804 CrossRef CAS PubMed.
- P. Angenendt, Drug Discovery Today, 2005, 10, 503 CrossRef CAS PubMed.
- J. Satija, V. V. R. Sai and S. Mukherji, J. Mater. Chem., 2011, 21, 14367 RSC.
- J. Ji, J. A. Schanzle and M. B. Tabacco, Anal. Chem., 2004, 76, 1411 CrossRef CAS PubMed.
- M. J. A. Shiddiky, M. A. Rahman and Y.-B. Shim, Anal. Chem., 2007, 79, 6886 CrossRef CAS PubMed.
- R. Benters, C. M. Niemeyer and D. Wöhrle, ChemBioChem, 2001, 2, 686 CrossRef CAS PubMed.
- R. K. Tekade, P. V. Kumar and N. K. Jain, Chem. Rev., 2009, 109, 49 CrossRef CAS PubMed.
- P. K. Maiti, T. Çağın, G. Wang and W. A. Goddard, Macromolecules, 2004, 37, 6236 CrossRef CAS.
- M. Hong, H. C. Yoon and H. Kim, Langmuir, 2003, 30, 416 CrossRef.
- D. A. Tomalia, K. Mardel, S. A. Henderson, G. Holan and R. Esfand, in Handbook of Nanoscience, Engineering, and Technology, ed. A. G. I. William, W. B. Donald, E. L. Sergey and J. I. Gerald, CRC Press, 2nd edn, 2003, pp. 20–1 Search PubMed.
- F. Zeng and S. C. Zimmerman, Chem. Rev., 1997, 97, 1681 CrossRef CAS PubMed.
- M. A. Mintzer and M. W. Grinstaff, Chem. Soc. Rev., 2011, 40, 173 RSC.
- A. D'Emanuele and D. Attwood, Adv. Drug Delivery Rev., 2005, 57, 2147 CrossRef PubMed.
- J. Majoros, A. Myc, T. Thomas, C. B. Mehta and J. R. Baker, Biomacromolecules, 2006, 7, 572 CrossRef PubMed.
- R. Menjoge, R. M. Kannan and D. A. Tomalia, Drug Discovery Today, 2010, 15, 171 CrossRef PubMed.
- E. Trévisiol, V. L. Berre-Anton, J. Leclaire, G. Pratviel, A. M. Caminade, J. P. Majoral, J. M. François and B. Meunier, New J. Chem., 2003, 27, 1713 RSC.
- P. K. Ajikumar, J. K. Ng, Y. C. Tang, J. Y. Lee, G. Stephanopoulos and H.-P. Too, Langmuir, 2007, 23, 5670 CrossRef CAS PubMed.
- G. Bar, S. Rubin, R. W. Cutts, T. N. Taylor and T. A. Zawodzinski, Langmuir, 1996, 12, 1172 CrossRef CAS.
- M. Schlupp, T. Weil, A. J. Berresheim, U. M. Wiesler, J. Bargon and K. Müllen, Angew. Chem., Int. Ed., 2001, 40, 4011 CrossRef CAS.
- P. Singh, T. Onodera, Y. Mizuta, K. Matsumoto, N. Miura and K. Toko, Sens. Actuators, B, 2009, 137, 403 CrossRef CAS.
- V. Zucolotto, A. P. A. Pinto, T. Tumolo, M. L. Moraes, M. S. Baptista, A. Riul, A. P. U. Araújo and O. N. Oliveira, Biosens. Bioelectron., 2006, 21, 1320 CrossRef CAS PubMed.
- J. Satija and S. Mukherji, Appl. Nanosci., 2012, 2, 293 CrossRef CAS.
- J. Cras, C. A. Rowe-Taitt, D. A. Nivens and F. S. Ligler, Biosens. Bioelectron., 1999, 14, 683 CrossRef CAS.
- P. Bhagwati, Ph.D. thesis, IIT Bombay, 2009.
- S. Pathak, A. K. Singh, J. R. Mcelhanon and P. M. Dentinger, Langmuir, 2004, 20, 6075 CrossRef CAS PubMed.
- V. Gajbhiye and N. K. Jain, Biomaterials, 2011, 32, 6213 CrossRef CAS PubMed.
- E. Peerce and E. M. Wright, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 2223 CrossRef.
- R. Suri and G. C. Mishra, Biosens. Bioelectron., 1996, 11, 1199 CrossRef PubMed.
- G. D. Nagare and S. Mukherji, Appl. Surf. Sci., 2009, 255, 3696 CrossRef CAS.
- S. Lord, M. Foss and F. Besenbacher, Nano Today, 2010, 5, 66 CrossRef.
- H. Ogi, Y. Fukunishi, H. Nagai, K. Okamoto, M. Hirao and M. Nishiyama, Biosens. Bioelectron., 2009, 24, 3148 CrossRef CAS PubMed.
- G. Kurth and T. Bein, Langmuir, 1993, 9, 2965 CrossRef.
- S. Hong, P. R. Leroueil, I. J. Majoros, B. G. Orr, J. R. Baker and M. M. B. Holl, Chem. Biol., 2007, 14, 107 CrossRef CAS PubMed.
- B. Girginer, B. Karagoz, M. Urgen and N. Bicak, Surf. Coat. Technol., 2008, 202, 4176 CrossRef CAS.
- N. Bui, M. Thompson, N. B. Mckeown, A. D. Romaschin and P. G. Kalman, Analyst, 1993, 118, 463 RSC.
- Z. Xu, Q. Liu and J. A. Finch, Appl. Surf. Sci., 1997, 120, 269–278 CrossRef CAS.
- E. Metwalli, D. Haines, O. Becker, S. Conzone and C. G. Pantano, J. Colloid Interface Sci., 2006, 298, 825 CrossRef CAS PubMed.
- Z. Nazarpoor, S. Ma, P. T. Fanson, O. S. Alexeev and M. D. Amiridis, Polym. Degrad. Stab., 2012, 97, 439 CrossRef CAS.
- A. J. Wagner, G. M. Wolfe and D. H. Fairbrother, Appl. Surf. Sci., 2003, 219, 317 CrossRef CAS.
- Y. Lee, J. Kim, S. Kim, W. D. Jang, S. Park and W. G. Koh, J. Mater. Chem., 2009, 19, 5643 RSC.
- S. S. Mark, N. Sandhyarani, C. Zhu, C. Campagnolo and C. A. Batt, Langmuir, 2004, 20, 6808 CrossRef CAS PubMed.
- R. Benters, C. M. Niemeyer, D. Drutschmann, D. Blohm and D. Wöhrle, Nucleic Acids Res., 2002, 30, E10 CrossRef PubMed.
- A. Leung, K. Rijal, P. M. Shankar and R. Mutharasan, Biosens. Bioelectron., 2006, 21, 2202 CrossRef CAS PubMed.
- P. V. Preejith, C. S. Lim, A. Kishen, M. S. John and A. Asundi, Biotechnol. Lett., 2003, 25, 105 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Calibration curve of FITC in tri-sodium phosphate buffer solution; algorithm used for the average height calculation and peak detection for AFM images in MATLAB 6; surface area calculations for the sensor matrix; absorbance spectra of a FITC-HIgG antibody solution (conc. = 100 μg mL−1); time-resolved absorbance response obtained from rabbit IgG coated sensor matrices when incubated with a nonspecific analyte. See DOI: 10.1039/c4ra00198b |
| This journal is © The Royal Society of Chemistry 2014 |