Ultra-small gold nanoparticles synthesized in aqueous solution and their application in fluorometric collagen estimation using bi-ligand functionalization†
Abstract
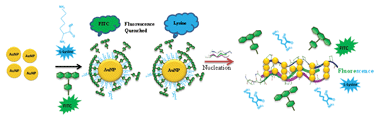
An effective, rapid and facile hydrosol approach was developed to synthesize monodisperse ultra-small gold nanoparticles (∼2 nm) using lithium borohydride (LiBH4) as a reducing agent. These lithium borohydride gold nanoparticles (LBH-AuNPs) are highly stable at pHs ranging from 3 to 10.6. We have subjected these LBH-AuNPs to bi-ligand co-functionalization with FITC (fluorescent isothiocyanate) and fluorescent lysine molecules. It was observed that these particles exhibit enhanced tolerance of FITC and lysine bi-ligand functionalization. The fluorescence resonance energy transfers (FRET) from the FITC and lysine to the AuNPs, and the replacement of these fluorophores by collagen as ligands have been exploited for the sensitive fluorometric detection of rat tail collagen. Linearity in the reappearance of FITC and lysine fluorescence was observed at 2 to 10 μg ml−1 of extracted rat collagen, which demonstrated the successful use of these bi-ligand surface functionalised gold nanoparticles as a probe for the sensitive fluorometric estimation of rat tail collagen.

 Please wait while we load your content...
Please wait while we load your content...