Heterocyclic terpenes: linear furano- and pyrroloterpenoids
Bin Wangab,
Lishu Wangac,
Yinglei Liac and
Yonghong Liu*a
aCAS Key Laboratory of Tropical Marine Bio-resources and Ecology/Guangdong Key Laboratory of Marine Materia Medica/RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, 510301, China. E-mail: yonghongliu@scsio.ac.cn; Fax: +86-20-89023244; Tel: +86-20-89023244
bShenzhen Shajing Affiliated Hospital of Guangzhou Medical University, Shenzhen 518104, China
cJilin Provincial Academy of Chinese Medicine Sciences, Changchun 130021, China
First published on 17th February 2014
Abstract
This review of furano- and pyrroloterpenoids covers the literature, 180 articles in all, published from January 2006 to December 2013. The relevant biological activities, source organisms, country of origin, and first total syntheses that lead to the revision of structures or stereochemistries of the compounds are described.
1. Introduction
This review of the literature from 2006 to 2013 covers the new occurrence of furano- and pyrroloterpenoids and describes 253 compounds in all from 180 articles.1 A number of reviews have dealt with natural sesquiterpenoids,2–7 diterpenoids,8–11 sesterterpenoids,12,13 triterpenoids,14–18 carotenoids and polyterpenoids.19–23 Other general reviews are: “Marine natural products”.24–30 In this review, we show structures of linear furano- and pyrroloterpenoids, and previously reported linear furano- and pyrroloterpenoids where there has been a structural revision or a newly established stereochemistry, previously reported linear furano- and pyrroloterpenoids for which first syntheses, new bioactivities, or new sources are described are referenced, and this review also added the furanotetraterpenoids and furanocarotenoids.Linear furano- and pyrroloterpenoids constitute small subgroups in which a terpenoid backbone are connected to differently substituted furan or pyrrole rings.31 Functional groups furan and its derivatives were widespread in natural products from terrestrial to marine organisms and irrespective of an isoprenic or polyketide origin, has been purported to possess enzyme-inhibiting functions.32 Molecules with antifouling activity represent a number of types including alkaloids, terpenoids, lactones, and pyrroles.33 Butenolide (furan-2(5H)-one) inhibited marine fouling.34,35 The butenolides were most likely to inhibit QS systems, since various butenolides are known to antagonize QS systems.36 It has been demonstrated that the furanone scaffolds inhibit formation of bacterial films by inhibiting quorum sensing through accelerated LuxR turnover.37 In structure–activity studies of limonin, it has been determined that the furan ring in the citrus limonoid structure are critical for the antifeedant activity of the limonoids against Colorado potato beetle larvae.38 Plants appear to use lactones to prevent being eaten and to avoid biofouling by bacteria while some animals use lactones to regulate their biochemical processes.32 Pyrolle skeleton looks like an enhancer of the deterrent activity exerted by the pyrrole counterpart.39
2. Furanoterpenoids
2.1. Furanosesquiterpenoids
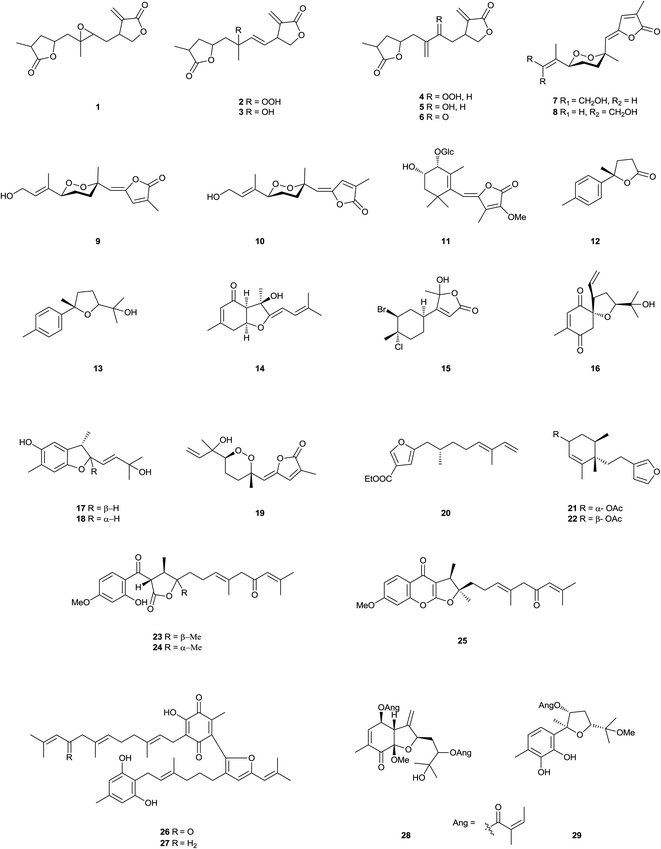
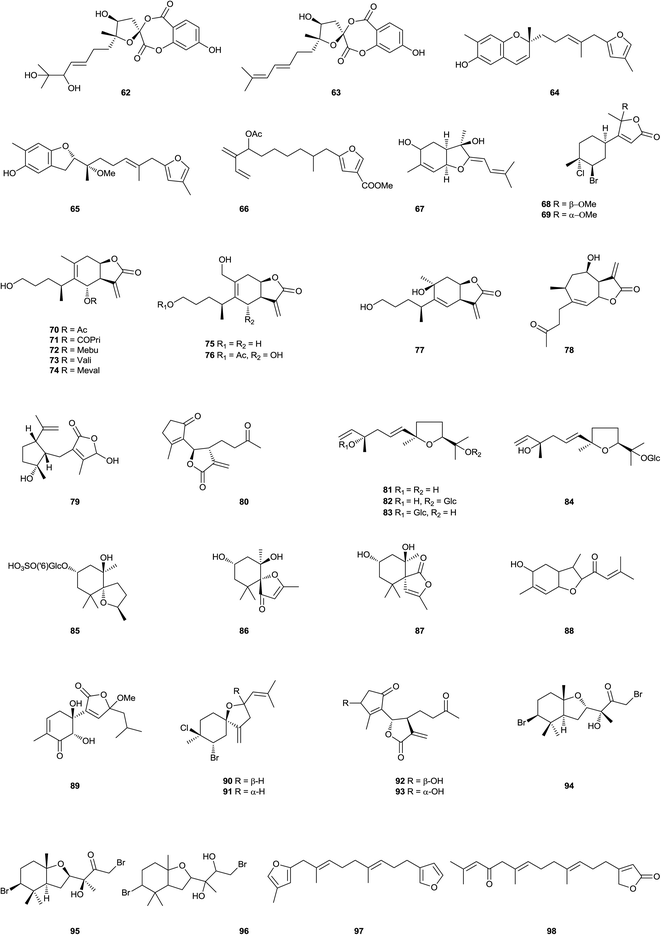
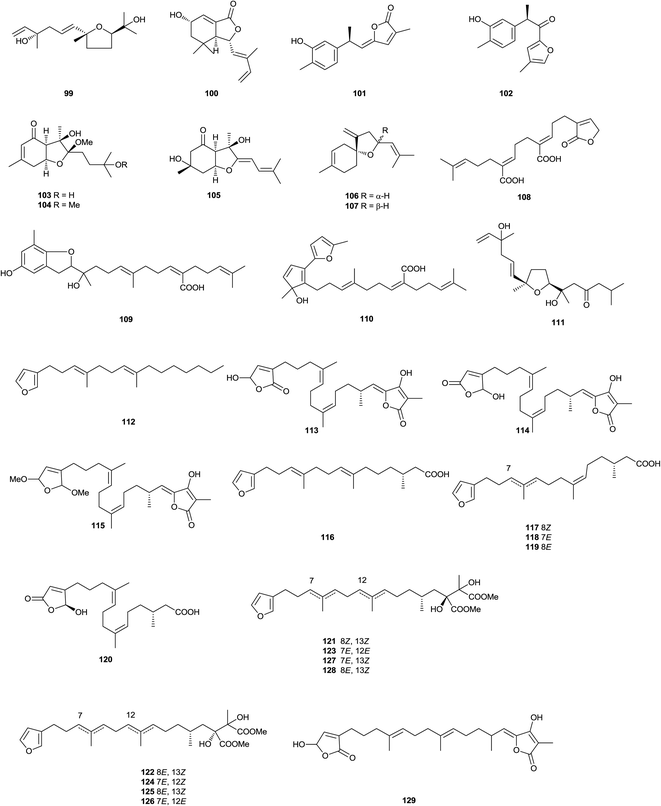
Five sesquiterpene lactones 1–5, and the known antheindurolide B, have been found in an extract from the aerial part of Anthemis arvensis (Kladovo, Serbia).40 The structure originally proposed of antheindurolide B has been revised to 6. Four sesquiterpene peroxides 7–10 have been obtained from a soft coral of the Sinularia genus (Northeastern coast of Taiwan).41 Whilst vignoside 11, a glucoside that inhibits the growth of human stomach cancer, has been obtained from Japanese adzuki bean Vigna angularis.42 The bisabolane derivatives boivinianins A 12 and B 13 have been identified as components of the stem bark of Cipadessa boiviniana (Northwest area of Madagascar).43 Whilst bisabolangelone 14, isolated from Angelica koreana (Cheongju, Korea), inhibits the production of melanin in α-melanocyte stimulating hormone-activated B16 melanoma cells or melan-a cells.44 The acaricidal active principle of an extract from the roots of Ostericum koreanum (Seoul, Korea)45 which affected adults of the Dermatophagoides genus, has been identified as the known sesquiterpene bisabolangelone 14. The sesquiterpenoids 15 have been found in an extract from the sea hare Aplysia dactylomela (La Palma, Canary Islands).46 Heliespirone C 16 was a sesquiterpene with two spiro heterocyclic skeleton, which have been isolated from Helianthus annuus (Junta de Andalucia, Jerez, Spain).47 The levels of activity shown by heliespirone C (56% inhibition) at 10−3 M in the coleoptiles bioassay, relative to controls, suggest that they may be lead compounds for agrochemicals. The structures of heliannuols G and H also obtained from this species, have been revised to 17 and 18, respectively, as a consequence of enantiocontrolled total syntheses.48 Two sesquiterpenes, sinularioperoxide E 19 and the ethoxy ester 20 were reported from a collection of soft coral Sinularia sp. (Northern east coast, Taiwan).49 Stable-isotope precursor feeding studies using Doriopsilla sp. (Arflor, Setubal, Portugal) established that a drimane ester mixture and 15-acetoxy-ent-pallescensin50 were derived de novo via the classical mevalonate pathway, that serve as defensive compounds to deter predation.51 The acetyl pelseneeriol-1 21 and acetyl pelseneeriol-2 22 have been obtained from the marine nudibranch Doriopsilla pelseneeri.51 The study also led to the characterisation of the 21 and 22. A study of the aerial parts and the roots of Dorema kopetdaghense (Khor Valley, Khorasan Razavi Province, Iran)52 afforded the sesquiterpene derivatives kopetdaghins C–E 23–25. A farnesyl phenol, grifolinone B 26, with inhibitory activity against nitric oxide production, has been found in a methanolic extract of the inedible mushroom Albatrellus caeruleoporus (Okutama, Tokyo, Japan).53 A further species of this genus, A. flettii (Bamfield, Vancouver Island, Canada),54 contains another dimeric merosesquiterpenoid, albatrinellin 27, together with the 16-alcohol corresponding to the ketone grifolinone B 26. Studies of an extract from the roots of Ligularia dentate (Sendai City, Miyagi Prefecture, Japan)55,56 afforded bisabolane sesquiterpenes 28 and 29. The carabrane derivative 30, has been obtained from Carpesium lipskyi (Mahan Mountain, Gansu Province, China).57 The furanosesquiterpene 31 has been identified as a component of Ximenia Americana.58 Flowers of Anthemis auriculata (Kresna, Bulgaria)59 contain seven linear sesquiterpene lactones 32–38. The furanosesquiterpene 39 has been found in the soft coral Sinularia asterolobata (Bali, Indonesia).60 A furanosesquiterpene isomicrocionin-3 40 has been isolated from a marine sponge of the Fasciospongia genus (Berlengas Islands, Portugal),61 whilst lingshuiolides A 41 and B 42, and lingshuiperoxide 43 have been found in an extract of the sponge Dysidea septosa (Lingshui Bay, Hainan Province, China).62 A seco-lactarane sesquiterpene, strobiluric acid 44, has been obtained from liquid cultures of the fungus Strobilurus stephanocystis (Chiba, Japan).63 A farnesane derivative 45 has been isolated from the aerial parts of Senecio cannabifolius (Changbai Mountain, Jilin Province, China).64 The nerolidane sesquiterpene 46 has been isolated from the seeds of Amomum xanthioides (Seoul, Korea). Compound 46 exhibited cytotoxicity against SK-OV-3 and SK-MEL-2 cells at IC50 values 16.7 and 8.6 μM, respectively, using a SRB bioassay.65 Another nerolidane derivative 47 and its glucoside 48 have been found in an extract from the leaves of Crataegus pinnatifida (Liaoning Province, China).66 Dalberpene 49 was a sesquiterpene, which has been obtained from the stems of Dalbergia parviflora (Bangkok, Thailand).67 The Indian soft coral Sinularia kavarattiensis (Gulf of Mannar, Tamil Nadu, India) contained the sesquiterpene 50, which showed antifouling activity against Balanus amphitrite at EC50 value 11.21 μg mL−1.68 3,4-Dehydrotheaspirone 51 has been obtained from the leaves of Juniperus brevifolia (Pedreira, Nordeste, São Miguel, Azores, Portugal).69 Xylacarpins D 52 and E 53 were two bisabolane derivatives, which have been obtained from cultures of the fungus Xylaria carpophila (Gaoligong Mountain, Yunnan Province, China).70 Chromatography of an extract from the fermentation of Trichoderma sp. PR-35 led to the isolation of the bisabolane derivative 54. This endophytic fungus have been obtained from Paeonia delavayi (Songming County, Yunnan Province, China).71 The sesquiterpenoid aspergillusene B 55 has been found in cultures of a sea fan Annella sp. (Coastal area in Suratthani Province, Thailand) derived strain of the fungus Aspergillus sydowii.72 Whilst two ar-bisabol derivatives 56 and 57 have been obtained from the stem bark of Fraxinus sielboldiana (Lu Mountain, Jiangxi Province, China).73 The stem bark of Illicium difengpi (Beijing, China)74 contained the sesquiterpene lactone 58. The sesquiterpene glycoside 59 has been found in extract of Breynia fruticosa (Nanning, Guangxi Province, China).75 A lactone (6R)-dehydroxysipandinolide (60), probably derived from a germacrane sesquiterpene, has been isolated from the rhizomes of Curcuma wenyujin (Wenzhou, Zhejiang Province, China).76 Neroplofurol 61 was a nerolidol derivative, which has been found in the inner stem bark of Oplopanax horridus (Alaska, USA).77 The roots of Ferula ferulaeoides (Shawan, Xinjiang Province, China) contained two sesquiterpenes, ferulactones A 62 and B 63.78 A chemical study of the soft coral Sinularia capillosa (Dongsha Atoll, Taiwan)79 afforded three sesquiterpenoids, named capillobenzopyranol 64, capillobenzofuranol 65, and capillofuranocarboxylate 66. 64 exhibited weak cytotoxicty against P-388 with ED50 values of 12.7 μM, 65 exhibited antiviral activity with IC50 13.5 μM, 64 significantly inhibited iNOS protein (36.7%) expression by LPS stimulation. Ashitabaol A 67 was an antioxidative sesquiterpene, which has been obtained from the seeds of Angelica keiskei (Nagahama, Japan).80 Red alga Laurencia catarinensis (Ilha do Arvoredo, Santa Catarina, Brazil), contained halogenated metabolites 68 and 69, which possessed cytotoxic properties.81 The eudesmanolides 70–77, which have been obtained from Inula japonica (Anhui Province, China).82,83 The seco-guaianolides 78–80, isolated from I. linearifolia (Changfeng County, Anhui Province, China),84 Chloranthus anhuiensis (Hexiang County, Anhui Province, China), Artemisia anomala (Hangzhou, Zhejiang Province, China),85–87 exhibited very weak antifungal activity.88 Schensianol A 81, schensianolsides A 82 and B 83 were obtained from the aerial parts of Euonymus schensianus (Luanchuan County, Henan Province, China).89 Another compound of this type 84 has been found in an extract from the roots of Clerodendrum bungei (Nanning, Guangxi Province, China) compounds were found to be moderately active to inhibit the proliferation of HeLa cells with the IC50 values 4.5 μM.90 A megastigmane sulfonoglucoside, anisoposide B 85, has been isolated from Mallotus anisopodus.91 Extraction of a marine sponge of the Spheciospongia genus (Sanya, Hainan Province, China),92 led to the isolation of spheciospongones A 86 and B 87. Chromatography of an extract from the rhizomes of Curcuma longa (Chongzhou, Sichuan Province, China)93 led to the isolation of the sesquiterpene 88. Whilst a study of the roots of Ginkgo biloba (Nanjing, Jiangsu Province, China) afforded bilobanol 89, a sesquiterpene trilactone.94 Two halogenated sesquiterpenes 90 and 91 have been found in an extract from the marine red alga Laurencia saltoi (Rongcheng, Shandong Province, China).95 From a Tanacetum parthenium extract afforded the epimeric iso-seco-tanapartholides 92 and 93, whose structures were determined by chemical synthesis using (−)-α-santonin as starting material, these sesquiterpene lactones proved to be inhibitors of the NF-κB signaling pathway.96 Three sesquiterpenes 94–96 have been isolated from Laurencia luzonensis (Sesoko Island, Okinawa).97 Two studies on green alga Bifucaria bifurcata (Roscoff, France) defined the metabolic makeup of this alga with the isolation of the sesquiterpenoids bibifuran 97 and eleganolone derivative 98.98,99 The furanosesquiterpenoid negunfurol 99 has been obtained from the seed of Vitex negundo (Wanglang National Nature Reserve, Sichuan Province, China).100 Hostasolide A 100 is a new lactone, which has been isolated from Hosta ensata (Linjiang City, Jilin Province, China).101 Abiesesquine A 101 and abiesesquine B 102 are two bisabolane sesquiterpenoids, which have been isolated from Abies holophylla (Huairen village, Benxi city, Liaoning Province, China).102 A bio-activity guided chromatography of an extract of Angelica koreana (Seoul, Korea)103 roots afforded three bisabolane derivatives, osterivolones A 103, B 104, and D 105. Okamurenes C 106 and D 107 have been obtained from the red alga Laurencia okamurai (Weihai coastline, Shandong Province, China).1042.2. Furanoditerpenoids
The insect growth regulatory activity of linear diterpenoids such as 108 obtained from Baccharis thymifolia (Villavicencio, Mendoza Province, Argentina) has been investigated.105 Thunbergol B 109 was isolated from Sargassum thunbergii (Busan, Korea) and was scavenger of the DPPH radical and of ONOO− from morpholinosydnonimine (SIN-1).106 In another large survey, 342 species of marine alga were screened against the bacterium Propionibacterium acnes, and the bacteriostatic compound sargafuran 110 was isolated from Japanese marine alga Sargassum macrocarpum. Sargafuran 110 suggested to be of geranylgeraniol/shikimate origin, had low cytotoxicity and could be the basis of a new skin care treatment to prevent or improve acne.107 Examination of a Huea species of antarctic lichen has led108 to the isolation of hueafuranoid A 111 which has inhibitory action against protein tyrosine phosphatase 1B, a system which is part of the cellular signal transduction cascade.2.3. Furanosesterterpenoids
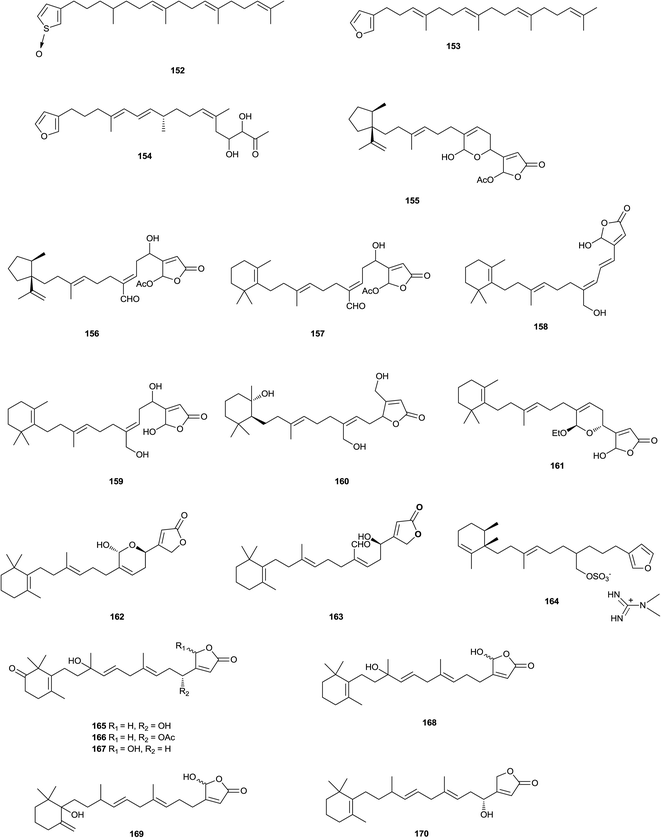
A C21 norfuranosesterterpenoid 112 was isolated from Lendenfeldia sp. (Indonesia).109 A series of furanosesterterpenoids 113–128 were isolated from a Sarcotragus sp. (Soheuksan Island, Korea), 126 and 127 were antibacterial, 117 and 118 were cytotoxic, while 120 was inhibitory towards isocitrate lyase. The absolute configurations of 129,110 previously reported as a synthetic analogue, and 116 (Sarcotragus sp.)111 were also assigned.112 The furanosesterterpenoid esters 130–132 were isolated from Coscinoderma mathewsi (Mooloolaba, Queensland, Australia).113 The norsesterterpenoids irciformonins E–K 133–139 were isolated from Ircinia formosana (East of Taiwan), of which irciformonin I 137 was found to inhibit peripheral blood mononuclear cell proliferation. In the same study irciformonin A (I. formosana)114 was re-isolated and the structure revised to 140.115 Palinurin (I. variabilis)116 has been synthesized.117 It was a non-ATP competitive glycogen synthase kinase 3β inhibitor. Antifouling activity has been reported118 for the sesterterpenoid (7E,12E,20Z)-variabilin (Sarcotragus sp.).111 Two oxidized sesterterpenoids 141 and 142 were isolated from the Mediterranean Spongia officinalis (Sicily coast, Mazara del Vallo, Italy).119 Six acyclic manoalide-related sesterterpenoids, hippolides C–H 143–148 were isolated from the South China Sea sponge Hippospongia lachne (Yongxing Island, Hainan Province, China). 145 showed weak anti-inflammatory activity, with IC50 value of 40.35 μM for PKCγ and PKCR, respectively.120 One C21 norsesterterpenoidal natural product, ircinolin A 149, two C22 furanosesterterpenoids, 15-acetylirciformonin B 150, and 10-acetylirciformonin B 151 were isolated from the sponge Ircinia sp. (Orchid Island, Taiwan). 150 and 151 exhibited significant cytotoxic activity against K562, DLD-1, HepG2, and Hep3B cancer cell lines.121 Bioactivity-guided fractionation of the ethyl acetate extract of a marine sponge Xestospongia sp. (Sikao Sea, Trang Province, Thailand), led to the isolation of a thiophene-S-oxide acyclic sesterterpenoid 152, which showed weak cytotoxicity against Vero cells.122 Furospinosulin-1 153, a marine sponge-derived furanosesterterpenoid, exhibited selective antiproliferative activity against DU145 human prostate cancer cells under hypoxic conditions. 153 also demonstrated antitumour activity at 10–50 mg kg−1 in oral administration to a mouse model inoculated with sarcoma S180 cells. Mechanistic analysis revealed that 153 suppresses transcription of the insulin-like growth factor-2 gene, which was selectively induced under hypoxic conditions through prevention of the binding of nuclear proteins to the Sp1 consensus sequence in the IGF-2 promoter region.123 A concise synthesis of 153 has been developed, and some structurally modified analogues were prepared. Biological evaluation of them revealed that the whole chemical structure was important for the hypoxia-selective growth inhibitory activity of 153.124 The norsesterterpenoid sarcotin P 154 has been isolated from the sponge Sarcotragus sp. (Cheju Island, Korea).125 Three acetylated sesterterpenoids, 25-acetoxyluffariellins A 155 and B 156, and 25-acetoxyseco-manoalide 157, were isolated from Luffariella variabilis (Orpheus Island, Australia). It was noted that the deacetylated versions were quickly formed if the sponge tissue was allowed to thaw before extraction, but only the acetylated compounds were isolated from the frozen sponge.126 The cytotoxic aplysinoplides A–C 158–160 were isolated from Aplysinopsis digitata (Oshima-shinsone, Kagoshima Prefecture, Japan).127 Manoalide (Luffariella variabilis),128 manoalide-25-acetate (Thorectandra excavatus),129 and secomanoalide (L. variabilis),130 inhibited quorum sensing in bacteria.131 24-Ethylmanoalide 161 was obtained from L. cf. variabilis (Mayotte Island, Indian Ocean). Since no ethanol was used in the isolation, 161 was presumed to be a natural product.132 The Great Barrier Reef sponge L. variabilis contained a range of secondary metabolites, including manoalide and manoalide monoacetate. As manoalide monoacetate has previously been shown to have antibacterial and quorum sensing inhibition activity, and was readily converted to manoalide, which also exhibited similar activity, the monoacetate may provide a chemical defence against predation and microbial attack.133 X-ray analysis of the crystalline product confirmed the structure and the reassignment of the absolute configuration of the structurally-related sponge metabolite alotaketal A 162.134 A sesterterpenoid, deoxymanoalide 163 was isolated from the nudibranch Chromodoris willani (Cape Zampa, Okinawa). The mollusk fed on a sponge containing manoalide and secomanoalide and was likely to biotransform them into 163. 163 showed moderate antimicrobial activity against Escherichia coli and Bacillus subtilis and inhibited snake venom phospholipase A2 at 0.2 to 0.5 μM.135 Rhabdastrella globostellata (Amami-Oshima, Kagoshima, Japan), yielded a monocyclic sesterterpenoid glycoside rhabdastoside A 164.136 The extract of marine sponge Hyrtios communis (The northern reefs region, Palau) was found to inhibit activation of the transcription factor hypoxia-inducible factor-1 (HIF-1) in T47D human breast tumor cells. Bioassay-guided isolation led to the identification of six (165–170) sesterterpenes. Two sesterterpenes, thorectidaeolide A 165 and 4-acetoxythorectidaeolide A 166 were among the most potent inhibitors of hypoxia (1% O2)-induced HIF-1 activation (IC50 values of 3.2 and 3.5 μM, respectively).137 The chemical investigation of the recently described Mediterranean Homoscleromorpha sponge Oscarella balibaloi (Marseilles, Maire Island and Frioul Island, France) revealed an original family of five closely related glucosylated sesterterpenes 171–174, named balibalosides. Balibalosides differ by the pattern of acetyl substitutions on the three sugar residues linked to the same aglycone sesterterpenoid core. From a biosynthetic perspective, these compounds may represent intermediates in the pathways leading to more complex sesterterpenes frequently found in Dictyoceratida, a sponge order belonging to Demospongiae, a clade which is phylogenetically distinct from the Homoscleromorpha. While steroid and triterpenoid saponins were already well known from marine sponges, balibalosides are the first examples of glycosilated sesterterpenes.1382.4. Furanotriterpenoids
Total synthesis of the pentacyclic triterpene polyether (+)-enshuol, originally isolated from Laurencia omaezakiana,139 established the absolute configuration as 175.140 The total synthesis of intricatetraol 176, a triterpene polyether metabolite of L. intricate,141 established the absolute configuration.142 Two polyether triterpenes, aplysiols A 177 and B 178, were isolated from the mantle of the sea hare Aplysia dactylomela (Hainan Island, China).143 The absolute stereochemistry of the pentanyltetrahydrofuran fragment of 177 was established and the remaining centers assigned to be the same as the structurally related co-metabolite (+)-thyrsiferol.144 A squalene-derived triterpene polyether, omaezakianol 179, was isolated from Laurencia omaezakiana Masuda (Enoshima, Japan) along with 180.145 The squalenoid-derived triterpenoid laurenmariannol 181 was isolated from L. mariannensis (Hainan and Weizhou Islands, China). Laurenmariannol 181 showed modest cytotoxic activity against P388 tumour cells.146 Spirodehydrovenustratriol 182 and 14-ketodehydrothyrsiferol 183 have been reported from the red alga L. viridis (Callao Salvaje, Paraiso Floral, Adeje, Tenerife, Canary Island).147 Aplysiols C–E 184–186 have been isolated from the marine alga Chondria armata (Hazard Bay, Orpheus Island, Queensland, Australia) together with the known aplysiol B whose structure has been revised to 187.148 Aplysqualenols A 188 and B 189, squalene-derived polyethers from the Caribbean sea slug Aplysia dactylomela (Bahia Salinas, Cabo Rojo, Puerto Rico), showed antiviral and antitumour activities.149 Aplysqualenol A 188 exhibited inhibitory activity against SNB-19 CNS cancer and T-47D breast cancer, with IC50 values of 0.4 and 0.3 μg mL−1, respectively. Aplysqualenol A 188 was very toxic against the Epstein–Barr virus in the VCA Elisa assay (EC90 = 0.08 μg mL−1) with no accompanying toxicity seen in the host Daudi cells. Compounds 188 and 189 showed moderate antiplasmodial activity against Plasmodium falciparum, with IC50 values of 11 and 18 μg mL−1, respectively. Ekeberins D2–D4 190–192 were antiplasmodial squalene derivatives from the stem bark of Ekebergia capensis (Mount Kenya Forest, Nanyuki area, Kenya).150 Three cytotoxic oxasqualenoids, 15-dehydroxythyrsenol A 193, prethyrsenol A 194, and 13-hydroxyprethyrsenol A 195, were isolated from Laurencia viridis (Paraiso Floral, Canary Islands).151 Molecular docking studies in the avb3 integrin binding region were used to explain their biological properties. From an earlier collection of L. viridis (Callao Salvaje, Paraiso Floral, Adeje, Tenerife, Canary Islands), iubol 196 and the venustatriol and thyrsiferol derivatives 197–199 were isolated as moderately cytotoxic compounds.152 The in vitro cytostatic activity of 196–199 was assessed by XTT assays, using several human cancer cell lines, including Jurkat (human T-cell acute leukemia), MM144 (human multiple myeloma), HeLa (human cervical carcinoma), and CADO-ES1 (human Ewing's sarcoma). Jurkat leukemic cells were the most sensitive cells to the tested polyether compounds. In particular, iubol 196, 22-hydroxy-15(28)-dehydrovenustatriol 197, and secodehydrothyrsiferol 199 showed the highest effectiveness against these cell lines (IC50, 2.0–3.5 μM). It was also noteworthy that all the above compounds were active against the CADO-ES1 cell line in the range of 10–12 μM.2.5. Furanotetraterpenoids
Two high molecular weight ether lipids, C151 and C153 lycopanerols H 200 and 201, have been isolated from the lipid extract of a strain of the green microalga Botryococcus braunii (Yamoussoukro, Ivory Coast). 200 and 201 arise from the linkage via ether bridges of tetraterpenoid, n-alkylphenol and α-tocopherol units.153 Lycopanerols H 200 and 201 could play a role in the prevention of oxidative damage to lipids in the L strains of B. braunii.2.6. Furanocaroteoids
Peridinin, a nor-carotenoid, exhibits an exceptionally high energy transfer efficiency to chlorophyll A in photosynthesis in the sea. This efficiency would be related to the unique structure of peridinin.154 Peridinin serves as photosynthesis accessory light harvesting pigment and extends the range of absorption for light-harvesting. It was associated with a protein and chlorophyll a and protects the light-harvesting pigments against photochemical damage caused by singlet molecular oxygen. All-trans-peridinin 202 has been isolated from Litophyton arboreum (Red Sea, Hurghada, Egypt) as well as Sarcophyton ehrenbergi (Bali, Indonesia), 202 was the characteristic major carotenoid of dinoflagellates from symbionts (zooxanthellae) of corals with a C37 skeleton; it contains six stereogenic centers and several functional groups. The configurational assignment of 202 as 3S,5R,6R,3′S,5′R,6′S was established by CD investigations. 202 was tested for antiproliferative activity against the cell lines HUVEC and K-562 (IC50 48.4, 53.8 μM), and for cytotoxicity against the cell line HeLa (IC50 51.9 μM), and showed moderate activities.155 202 presents a difficult challenge to the synthetic chemist. A method that involves a Wittig reaction between the appropriate conjugated phosphanylidenebutenolides and the C10 or C15 aldehyde provided an efficient route to the model compounds.156 202 from a dinoflagellate have been described.157 The stereochemistry of furanoid derivative sinensiachrome (3S,5R,8S)-5,8-epoxy-5,8-dihydro-10′-apo-β-carotene-3,10′-diol 203 has been established.158 Re-examination of persicaxanthin and persicachrome, from cling peaches, confirmed the structure of persicachrome as 204.159 Syntheses of the acetylenic and allenic C22-apocarotenals and proof that were related to peridinin and pyrrhoxanthin (5′,6′-epoxy-3,3′-dihydroxy-7,8-didehydro-5′,6′-dihydro-10,11,20-trinor-β,β-caroten-11′,19′-olide 3-acetate) 205 were achieved by a C15 + C7 reaction.160 An apoviolaxanthinol structure has been derived for persicaxanthin, from the French plum, which also yielded the corresponding 5,8-furanoid oxide persicachrome 206.161 HPLC methods have been devised for separating diastereoisomeric and epimeric carotenoid furanoid oxides, and analysis of the purified products has allowed determination of the absolute configurations of and the diastereoisomeric mutatoxanthins 5,8-epoxy-5,8-dihydro-β,β-carotene-3,3′-diols 207 (ref. 162) and of the neochromes 5′,8′-epoxy-6,7-didehydro-5,6,5′,8′-tetrahydro-β,β-carotene-3,5,3′-triols 208.163 Eugster and his co-workers have synthesized the diastereoisomeric (5R,8R,5′R,8′R)-, (5R,8S,5′R,8′S)-, and (5R,8R,5′R,8′S)-aurochromes 209–211, respectively, and the meso-forms (5R,8R,5′S,8′S)-and (5R,8S,5′S,8′R)-aurochrome 212 and 213 by condensation of the C10 Wittig salt with the appropriate isomer of the C15 aldehyde.164 Acid-catalyzed isomerization of (5S,6R)-5,6-epoxy-5,6-dhiydro-β,β-carotene similarly gave the 8-epimeric mutatochromes 214 and 215 (5S,8S)-and (5S,8R)-5,8-epoxy-5,8-dihydro-β,β-carotene, which were fully characterized by spectroscopic methods.165 Carotenoid epoxides are being found in increasing variety. Although xanthophyll epoxides were among the most common naturally occurring carotenoids, and carotene 5,6-epoxides have frequently been reported as minor constituents of carotenoid extracts, the identification of luteochrome (5R,6S,5′R,8′R)-5,6; 5′,8′-diepoxy-5,6,5′,8′-tetrahydro-β-β-carotene 216 in Brazilian sweet potatoes was the first report of the isolation of a carotenoid with an unhydroxylated 5,6-epoxy-5,6-dihydro-β-ring optically active form.166 One has the feature of a 4,5-epoxy-4,5-dihydro-ε-end-group and both have 7,8-dihydrostructures; this latter feature has previously only been reported in cyclic carotenoids isolated from animal sources. The compounds were characterized by spectroscopic methods, as (3S,3′R)4′,5′-epoxy-3,6,3′-trihydroxy-7,8,4′,5′,7′,8′-hexahydro-γ,ε,-caroten-one 217 and (3S,3′R)-5,6-epoxy-3,3′-dihydroxy-5,6,7′,8′-tetrahydro-β-β-caroten-11′,19′-olide 218. Detailed studies leading to the rigorous assignment of stereochemistry as (3S,6R,3′R,6′R) for prasinoxanthin itself have been presented.167 C33-, C35-, and C39-peridinins 219–221 were synthesized.154,168 A C37 carotenoid, isolated as a mixture of esters 222, were reported from the clam Paphia amabillis (Mimase, Japan).1693. Pyrroloterpenoids
3.1. Pyrrolosesquiterpenoids
Total synthesis of glaciapyrrole A from an Alaskan Streptomyces strain170 as well as of seven stereoisomers via a diastereoselective ruthenium-catalysed approach, from either geraniol or nerol, clarified the relative configuration of natural glaciapyrrole A and a subsequent enantioselective synthesis of the unnatural enantiomer determined the configuration of the natural product as (11R,12S,15S)-(+)-glaciapyrrole A 223.171 An actinomycete of the “MAR4” group (La Jolla, California, USA) was an excellent source of hybrid isoprenoid natural products,172 yielding nitropyrrolins A–E 224–228. Determination of the absolute configuration of nitropyrrolin B 225 via one-step acetonide formation from an epoxide, and application of the modified Mosher method afforded a general method for configurational assignment of trisubstituted epoxides. Nitropyrrolins A 224 and B 225 were moderately cytotoxic to HCT-116 cells, while nitropyrrolin D 227 was considerably more cytotoxic.173 Heronapyrroles A–C (ref. 174) (229–231, respectively) were cytotoxic farnesyl nitropyrroles which were isolated from saline cultures of marine-derived bacteria of the actinomycete family Streptomycetaceae, chemical analysis of a marine-derived Streptomyces sp. CMB-M0423 (Beach sand off Heron Island, Queensland, Australia) yielded three members of the rare pyrroloterpene biosynthetic structure class. Heronapyrroles A–C 229–231 displayed promising biological activities with low to submicromolar IC50 activity against Gram-positive bacteria but no cytotoxicity toward mammalian cell lines. They did display very promising activity against the Gram-positive bacteria Staphylococcus aureus ATCC 9144 (IC50 0.6–1.1 μM) and Bacillus subtilis ATCC 6633 (IC50 1.1–6.5 μM).174 The first synthesis of (−)-heronapyrrole C 231, the enantiomer of a unique farnesylated 2-nitropyrrole natural product is described. With none of the chiral centers of heronapyrrole C 231 originally assigned, we proposed the most likely natural configuration on the basis of a putative biosynthetic pathway. The key step of the synthesis is a biomimetic polyepoxide cyclization cascade to establish the bis-THF moiety. Thus, (−)-heronapyrrole C 231 was synthesized in eight steps from commercially available starting materials.31 While aspernidines A 232 and B 233 were two farnesyl isoindolinone-alkaloids, which have been found in an extract of the fungus Aspergillus nidulans.175 Another fungus Emericella sp. associated with Aegiceras corniculatum (Haikou, Hainan Province, China), contained the sesquiterpene-isoindolone ethers emeriphenolicins A–F 234–239.176 Chemical analysis of a specimen of the sponge Ianthella cf. flabelliformis (Lonsdale Wall, The Rip, Port Phillip Heads, Victoria, Australia) returned two new sesquiterpene glycinyl lactams, ianthellalactams A 240 and B 241.1773.2. Pyrrolosesterterpenoids
Hippolides A 242 and B 243 were manoalide derivatives from Hippospongia lachne (Yongxing Island, Hainan Province, China). Compound 242 exhibited cytotoxicity against A549, HeLa, and HCT-116 cell lines with IC50 values of 5.22 × 10−2, 4.80 × 10−2, and 9.78 μM, respectively. It also showed moderate PTP1B inhibitory activity with an IC50 value of 23.81 μM, and compound 243 showed moderate cytotoxicity against the HCT-116 cell line and PTP1B inhibitory activity with IC50 values of 35.13 and 39.67 μM, respectively. In addition, 242 showed weak anti-inflammatory activity, with IC50 values of 61.97 μM for PKCγ and PKCR, respectively.120 Six ircinialactam sesterterpenoids 244–249 have been reported from southern Australian and Antarctic collections of Ircinia sp. and Psammocinia sp. (Southern Australia and Antarctica) both from the family Irciniidae. All were isoform-selective glycine-gated chloride channel receptor modulators and have potential roles as neuronal pharmacological agents.178 A series of sesterterpenoids 250–253 were isolated from a Sarcotragus sp. (Soheuksan Island, Korea).1124. Conclusions
Terpenoids are the most diverse class of natural products and were of interest since they are found in almost all life forms where they carry out a myriad of functions ranging from primarily structural (cholesterol in cell membranes) to functional (carotenoids in photosynthesis, retinal in vision, quinones in electron transfer).179 Terpenoids have a variety of roles in mediating antagonistic and beneficial interactions among organisms. They defend many species of plants, animals and microorganisms against predators, pathogens and competitors, and they are involved in conveying messages to conspecifics and mutualists regarding the presence of food, mates and enemies.180Living organisms produce terpenoids for certain physiological and ecological functions. The idea that terpenoids have important biological functions has taken hold only recently, and there were considerable difficulties in testing these compounds in nature. Recent advances in analytical chemistry also help in functional studies by providing a much more comprehensive view of terpenoids present in and around individual organisms than was previously available. With the emerging discipline of chemical ecology, we will learn more about the roles of linear furano- and pyrroloterpenoids in the nature in the near future.
Acknowledgements
This study was supported by the National Basic Research Program of China (973 Program, no. 2010CB833800 and 2011CB915503), the National High Technology Research and Development Program (863 Program, 2012AA092104), the National Natural Science Foundation of China (no. 31270402, 21172230, 20902094, 41176148, and 21002110), Guangdong Province-CAS Joint Research Program (2011B090300023 and 2012B091100264), and Guangdong Marine Economic Development and Innovation of Regional Demonstration Project (GD2012-D01-001 and GD2012-D01-002).Notes and references
- Y. Liu, S. Zhang and P. J. M. Abreu, Nat. Prod. Rep., 2006, 23, 630 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2007, 24, 1350 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2008, 25, 1180 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2009, 26, 1125 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2010, 27, 1681 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2011, 28, 1580 RSC.
- B. M. Fraga, Nat. Prod. Rep., 2012, 29, 1334 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2012, 29, 890 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2011, 28, 1755 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2009, 26, 1156 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2007, 24, 1332 RSC.
- Y. Liu, L. Wang, J. H. Jung and S. Zhang, Nat. Prod. Rep., 2007, 24, 1401 RSC.
- L. Wang, B. Yang, X.-P. Lin, X.-F. Zhou and Y. Liu, Nat. Prod. Rep., 2013, 30, 455 RSC.
- R. A. Hill and J. D. Connolly, Nat. Prod. Rep., 2012, 29, 780 RSC.
- R. A. Hill and J. D. Connolly, Nat. Prod. Rep., 2011, 28, 1087 RSC.
- J. D. Connolly and R. A. Hill, Nat. Prod. Rep., 2010, 27, 79 RSC.
- J. D. Connolly and R. A. Hill, Nat. Prod. Rep., 2008, 25, 794 RSC.
- J. D. Connolly and R. A. Hill, Nat. Prod. Rep., 2007, 24, 465 RSC.
- G. Britton, Nat. Prod. Rep., 1984, 1, 67 RSC.
- G. Britton, Nat. Prod. Rep., 1985, 2, 349 RSC.
- G. Britton, Nat. Prod. Rep., 1986, 3, 591 RSC.
- G. Britton, Nat. Prod. Rep., 1989, 6, 359 RSC.
- G. Britton, Nat. Prod. Rep., 1991, 8, 223 RSC.
- J. W. Blunt, B. R. Copp, W.-P. Hu, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2007, 24, 31 RSC.
- J. W. Blunt, B. R. Copp, W.-P. Hu, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2008, 25, 35 RSC.
- J. W. Blunt, B. R. Copp, W.-P. Hu, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2009, 26, 170 RSC.
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2010, 27, 165 RSC.
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2011, 28, 196 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2012, 29, 144 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2013, 30, 237 RSC.
- J. Schmidt and C. B. W. Stark, Org. Lett., 2012, 14, 4042 CrossRef CAS PubMed.
- M. I. Konaklieva and B. J. Plotkin, Mini-Rev. Med. Chem., 2005, 5, 73 CrossRef CAS.
- H.-U. Dahms, X. Ying and C. Pfeiffer, Biofouling, 2006, 22, 317 CrossRef CAS PubMed.
- Y.-F. Zhang, H. Zhang, L. He, C. Liu, Y. Xu and P.-Y. Qian, ACS Chem. Biol., 2012, 7, 1049 CrossRef CAS PubMed.
- N. Fusetani, Nat. Prod. Rep., 2011, 28, 400 RSC.
- T. Persson, M. Givskov and J. Nielsen, Curr. Med. Chem., 2005, 12, 3103 CrossRef CAS.
- N. Fusetani, Nat. Prod. Rep., 2004, 21, 94 RSC.
- A. Roy and S. Saraf, Biol. Pharm. Bull., 2006, 29, 191 CAS.
- B. Forte, B. Malgesini, C. Piutti, F. Quartieri, A. Scolaro and G. Papeo, Mar. Drugs, 2009, 7, 705 CrossRef CAS PubMed.
- I. Vukovic, L. Vujisic, V. Vajs, V. Tesevic, S. Macura, P. Janackovic and S. Milosavljevic, Biochem. Syst. Ecol., 2006, 34, 303 CrossRef PubMed.
- C. H. Chao, C. H. Hsieh, S. P. Chen, C. K. Lu, C. F. Dai, Y. C. Wu and J. H. Sheu, Tetrahedron Lett., 2006, 47, 2175 CrossRef CAS PubMed.
- T. Itch, Y. Itoh, H. Hibasami, H. Katsuzaki, K. Imai, Y. Furuichi and T. Komiya, J. Jpn. Soc. Food Sci. Technol., 2005, 52, 319 CrossRef.
- D. A. Mulholland, K. McFarland and M. Randrianarivelojosia, Biochem. Syst. Ecol., 2006, 34, 365 CrossRef CAS PubMed.
- E. Roh, C.-Y. Yun, J. W. Lee, C. Lee, B. Y. Hwang, S. T. Oh, S.-H. Jung, S.-B. Han and Y. Kim, Planta Med., 2011, 77, 248 CrossRef CAS PubMed.
- S. W. Kang, H. K. Kim, W. J. Lee and Y. J. Ahn, J. Agric. Food Chem., 2006, 54, 3547 CrossRef CAS.
- I. Brito, T. Dias, A. R. Diaz-Marrero, J. Darias and M. Cueto, Tetrahedron, 2006, 62, 9655 CrossRef CAS PubMed.
- F. A. Macias, J. L. G. Galindo, R. M. Varela, A. Torres, J. M. G. Molinillo and F. R. Fronczek, Org. Lett., 2006, 8, 4513 CrossRef CAS PubMed.
- S. Morimoto, M. Shindo, M. Yoshida and K. Shishido, Tetrahedron Lett., 2006, 47, 7353 CrossRef CAS PubMed.
- J.-H. Su, C.-H. Hsieh, C.-L. Lo, C.-Y. Huang, C.-F. Dai, Y.-H. Kuo and J.-H. Sheu, J. Chin. Chem. Soc., 2008, 55, 1286 CAS.
- A. Spinella, L. A. Alvarez, C. Avila and G. Cimino, Tetrahedron Lett., 1994, 35, 8665 CrossRef CAS.
- H. Gaspar, A. Cutignano, T. Ferreira, G. Calado, G. Cimino and A. Fontana, J. Nat. Prod., 2008, 71, 2053 CrossRef CAS PubMed.
- M. Iranshahi, F. Shaki, A. Mashlab, A. Porzel and L. A. Wessjohann, J. Nat. Prod., 2007, 70, 1240 CrossRef CAS PubMed.
- D. N. Quang, T. Hashimoto, Y. Arakawa, C. Kohchi, T. Nishizawa, G. I. Soma and Y. Asakawa, Bioorg. Med. Chem., 2006, 14, 164 CrossRef CAS PubMed.
- B. Koch and W. Steglich, Eur. J. Org. Chem., 2007, 1631 CrossRef CAS.
- H. Baba, Y. Yaoita and M. Kikuchi, Helv. Chim. Acta, 2007, 90, 1028 CrossRef CAS.
- H. Baba, Y. Yaoita and M. Kikuchi, Helv. Chim. Acta, 2007, 90, 1302 CrossRef CAS.
- J. N. Wang, S. P. Gu and R. X. Tan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2007, 46, 985 Search PubMed.
- M. R. Silva de Araujo, J. C. da Costa Assuncao, I. N. Fernandes Dantas, L. V. Costa-Lotufo and F. J. Queiroz Monte, Nat. Prod. Commun., 2008, 3, 857 CAS.
- M. Todorova, J. Staneva, P. Denkova and L. Evstatieva, Nat. Prod. Res., 2008, 22, 907 CrossRef CAS PubMed.
- D. Grote, H.-M. Dahse and K. Seifert, Chem. Biodiversity, 2008, 5, 2449 CAS.
- H. Gaspar, S. Santos, M. Carbone, A. S. Rodrigues, A. I. Rodrigues, M. J. Uriz, S. M. S. Feio, D. Melck, M. Humanes and M. Gavagnin, J. Nat. Prod., 2008, 71, 2049 CrossRef CAS PubMed.
- X.-C. Huang, J. Li, Z.-Y. Li, L. Shi and Y.-W. Guo, J. Nat. Prod., 2008, 71, 1399 CrossRef CAS PubMed.
- F. Hiramatsu, T. Murayama, T. Koseki and Y. Shiono, Nat. Prod. Res., 2008, 22, 1007 CrossRef PubMed.
- W.-D. Xie, C.-W. Weng, X. Gao, H. Zhao and K.-H. Row, J. Chin. Chem. Soc., 2010, 57, 436 CAS.
- K. H. Kim, J. W. Choi, S. U. Choi and K. R. Lee, Nat. Prod. Sci., 2011, 17, 10 CAS.
- S.-J. Song, L.-Z. Li, P.-Y. Gao, Y. Peng, J.-Y. Yang and C.-F. Wu, Food Chem., 2011, 129, 933 CrossRef CAS PubMed.
- U. Songsiang, C. Hahnvajanawong and C. Yenjai, Fitoterapia, 2011, 82, 1169 CrossRef CAS PubMed.
- V. P. L. Mol, T. V. Raveendran, P. S. Parameswaran, R. J. Kunnath and N. Sathyan, Indian J. Mar. Sci., 2010, 39, 270 CAS.
- L. M. Moujir, A. M. L. Seca, L. Araujo, A. M. S. Silva and M. C. Barreto, Fitoterapia, 2011, 82, 225 CrossRef CAS PubMed.
- X. Yin, T. Feng, Z.-H. Li, J. Su, Y. Li, N.-H. Tan and J.-K. Liu, Nat. Prod. Bioprospect., 2011, 1, 75 CrossRef CAS.
- S.-H. Wu, L.-X. Zhao, Y.-W. Chen, R. Huang, C.-P. Miao and J. Wang, Chem. Biodiversity, 2011, 8, 1717 CAS.
- K. Trisuwan, V. Rukachaisirikul, M. Kaewpet, S. Phongpaichit, N. Hutadilok-Towatana, S. Preedanon and J. Sakayaroj, J. Nat. Prod., 2011, 74, 1663 CrossRef CAS PubMed.
- S. Lin, Y.-L. Zhang, M.-T. Liu, J.-C. Zi, M.-L. Gan, W.-X. Song, X.-N. Fan, S.-J. Wang, Y.-C. Yang and J.-G. Shi, Acta Pharm. Sin. B, 2011, 1, 89 CAS.
- L. Fang, X.-J. Wang, S.-G. Ma and S.-S. Yu, Acta Pharm. Sin. B, 2011, 1, 178 CAS.
- D. Meng, J. Wu and W. Zhao, Phytochemistry, 2010, 71, 325 CrossRef CAS PubMed.
- Y. Lou, F. Zhao, H. He, K.-F. Peng, L.-X. Chen and F. Qiu, Chem. Biodiversity, 2010, 7, 1245 CAS.
- T. Inui, Y. Wang, D. Nikolic, D. C. Smith, S. G. Franzblau and G. F. Pauli, J. Nat. Prod., 2010, 73, 563 CrossRef CAS PubMed.
- Y. Hu, X.-D. Li, G.-Y. Li, N. Lin, W.-J. Zuo, Y.-M. Zeng, H. Meng, X. Li and J.-H. Wang, Helv. Chim. Acta, 2010, 93, 1019 CrossRef CAS.
- S.-Y. Cheng, K.-J. Huang, S.-K. Wang, Z.-H. Wen, P.-W. Chen and C.-Y. Duh, J. Nat. Prod., 2010, 73, 771 CrossRef CAS PubMed.
- N. Aoki and S. Ohta, Tetrahedron Lett., 2010, 51, 3449 CrossRef CAS PubMed.
- C. Lhullier, M. Falkenberg, E. Ioannou, A. Quesada, P. Papazafiri, P. A. Horta, E. P. Schenkel, C. Vagias and V. Roussis, J. Nat. Prod., 2010, 73, 27 CrossRef CAS PubMed.
- J.-J. Qin, H.-Z. Jin, J.-X. Zhu, J.-J. Fu, Q. Zeng, X.-R. Cheng, Y. Zhu, L. Shan, S.-D. Zhang, Y.-X. Pan and W.-D. Zhang, Tetrahedron, 2010, 66, 9379 CrossRef CAS PubMed.
- J. J. Qin, H. Z. Jin, J. X. Zhu, J. J. Fu, X. J. Hu, X. H. Liu, Y. Zhu, S. K. Yan and W. D. Zhang, Planta Med., 2010, 76, 278 CrossRef CAS PubMed.
- L.-Y. Nie, J.-J. Qin, Y. Huang, L. Tan, Y.-B. Liu, Y.-X. Pan, H.-Z. Jin and W.-D. Zhang, J. Nat. Prod., 2010, 73, 1117 CrossRef CAS PubMed.
- K. Zan, S.-P. Shi, Q. Fu, X.-Q. Chen, S.-X. Zhou, M.-T. Xiao and P.-F. Tu, Helv. Chim. Acta, 2010, 93, 2000 CrossRef CAS.
- J. Wen, H. Shi, Z. Xu, H. Chang, C. Jia, K. Zan, Y. Jiang and P. Tu, J. Nat. Prod., 2010, 73, 67 CrossRef CAS PubMed.
- M. Zan, X.-Q. Chen, Q. Fu, S.-P. Shi, S.-X. Zhou, M.-T. Xiao and P.-F. Tu, Biochem. Syst. Ecol., 2010, 38, 431 CrossRef PubMed.
- Y.-J. Xu, C.-P. Tang, M.-J. Tan, C.-Q. Ke, T. Wu and Y. Ye, Chem. Biodiversity, 2010, 7, 151 CAS.
- X. K. Zheng, J. H. Guo, W. S. Feng and H. W. Li, Chin. Chem. Lett., 2009, 20, 952 CrossRef CAS PubMed.
- S.-S. Liu, T. Zhou, S.-W. Zhang and L.-J. Xuan, Helv. Chim. Acta, 2009, 92, 1070 CrossRef CAS.
- M. Chau Van, T. Nguyen Thi Kim, Q. Tran Hong, C. Nguyen Xuan, T. Nguyen Nghia, N. Nguyen Hoai, Y. Vander Heyden, J. Quetin-Leclercq and K. Phan Van, Nat. Prod. Commun., 2009, 4, 889 Search PubMed.
- D. Liu, M.-J. Xu, L.-J. Wu, Z.-W. Deng and W.-H. Lin, J. Asian Nat. Prod. Res., 2009, 11, 811 CrossRef CAS PubMed.
- W. Li, J.-T. Feng, Y.-S. Xiao, Y.-Q. Wang, X.-Y. Xue and X.-M. Liang, J. Asian Nat. Prod. Res., 2009, 11, 569 CrossRef PubMed.
- P. Yu and J. Y. Liang, Chin. Chem. Lett., 2009, 20, 1224 CrossRef CAS PubMed.
- N.-Y. Ji, X.-M. Li, K. Li and B.-G. Wang, Helv. Chim. Acta, 2009, 92, 1873 CrossRef CAS.
- E. F. Makiyi, R. F. M. Frade, T. Lebl, E. G. Jaffray, S. E. Cobb, A. L. Harvey, A. M. Z. Slawin, R. T. Hay and N. J. Westwood, Eur. J. Org. Chem., 2009, 5711 CrossRef CAS PubMed.
- D. S. Makhanu, M. Yokoyama, T. Miono, T. Maesato, M. Maedomari, P. Wisespongpand and M. Kuniyoshi, Bull. Fac. Sci., Univ. Ryukyus, 2006, 81, 115 CAS.
- Q. Goethel, J. Munoz and M. Koeck, Phytochem. Lett., 2012, 5, 693 CrossRef CAS PubMed.
- Q. Goethel, E. Lichte and M. Koeck, Tetrahedron Lett., 2012, 53, 1873 CrossRef CAS PubMed.
- C.-J. Zheng, J. Pu, H. Zhang, T. Han, K. Rahman and L.-P. Qin, Fitoterapia, 2012, 83, 49 CrossRef CAS PubMed.
- H.-X. Liu, Q.-Y. Sun, F.-M. Yang, F.-W. Zhao, Y.-H. Wang and C.-L. Long, Chem. Nat. Compd., 2012, 48, 580 CrossRef CAS.
- J.-H. Xia, S.-D. Zhang, Y.-L. Li, L. Wu, Z.-J. Zhu, X.-W. Yang, H.-W. Zeng, H.-L. Li, N. Wang, A. Steinmetz and W.-D. Zhang, Phytochemistry, 2012, 74, 178 CrossRef CAS PubMed.
- J. W. Lee, C.-Y. Yun, E. Roh, C. Lee, Q. Jin, K. K. Bang, S.-H. Jung, D. Lee, M. K. Lee, Y. Kim and B. Y. Hwang, Bioorg. Med. Chem. Lett., 2012, 22, 2927 CrossRef CAS PubMed.
- Y. Liang, X.-M. Li, C.-M. Cui, C.-S. Li, H. Sun and B.-G. Wang, Mar. Drugs, 2012, 10, 2817 CrossRef PubMed.
- V. E. J. Hikawczuk, J. R. Saad, O. S. Giordano, C. Garcia, T. Martin, V. S. Martin, M. E. Sosa and C. E. Tonn, J. Nat. Prod., 2008, 71, 190 CrossRef PubMed.
- Y. Seo, K. E. Park, Y. A. Kim, H.-J. Lee, J.-S. Yoo, J.-W. Ahn and B.-J. Lee, Chem. Pharm. Bull., 2006, 54, 1730 CrossRef CAS.
- Y. Kamei, M. Sueyoshi, K.-I. Hayashi, R. Terada and H. Nozaki, J. Antibiot., 2009, 62, 259 CrossRef CAS PubMed.
- Y. Cui, J. H. Yim, D.-S. Lee, Y.-C. Kim and H. Oh, Bioorg. Med. Chem. Lett., 2012, 22, 7393 CrossRef CAS PubMed.
- J. Dai, Y. Liu, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2007, 70, 1824 CrossRef CAS PubMed.
- C. J. Barrow, J. W. Blunt and M. H. G. Munro, J. Nat. Prod., 1989, 52, 346 CrossRef CAS.
- C. J. Barrow, J. W. Blunt, M. H. G. Munro and N. B. Perry, J. Nat. Prod., 1988, 51, 275 CrossRef CAS.
- N. Wang, J. Song, K. H. Jang, H.-S. Lee, X. Li, K.-B. Oh and J. Shin, J. Nat. Prod., 2008, 71, 551 CrossRef CAS PubMed.
- K. W. L. Yong, J. N. A. Hooper and M. J. Garson, ARKIVOC, 2008, 100 CrossRef CAS.
- Y.-C. Shen, K.-L. Lo, Y.-C. Lin, A. T. Khalil, Y.-H. Kuo and P.-S. Shih, Tetrahedron Lett., 2006, 47, 4007 CrossRef CAS PubMed.
- Y.-C. Shen, P.-S. Shih, Y.-S. Lin, Y.-C. Lin, Y.-H. Kuo, Y.-C. Koo and A. T. Khalil, Helv. Chim. Acta, 2009, 92, 2101 CrossRef CAS.
- G. Alfano, G. Cimino and S. Destefano, Experientia, 1979, 35, 1136 CrossRef CAS.
- M. Perez, D. I. Perez, A. Martinez, A. Castro, G. Gomez and Y. Fall, Chem. Commun., 2009, 3252 RSC.
- M. Stewart, C. Depree and K. J. Thompson, Nat. Prod. Commun., 2009, 4, 331 CAS.
- E. Manzo, M. L. Ciavatta, G. Villani, M. Varcanrionti, S. M. Abu Sayem, R. van Soest and M. Gavagnin, J. Nat. Prod., 2011, 74, 1241 CrossRef CAS PubMed.
- S.-J. Piao, H.-J. Zhang, H.-Y. Lu, F. Yang, W.-H. Jiao, Y.-H. Yi, W.-S. Chen and H.-W. Lin, J. Nat. Prod., 2011, 74, 1248 CrossRef CAS PubMed.
- J.-H. Su, S.-W. Tseng, M.-C. Lu, L.-L. Liu, Y. Chou and P.-J. Sung, J. Nat. Prod., 2011, 74, 2005 CrossRef CAS PubMed.
- P. Pedpradab and K. Suwanborirux, J. Asian Nat. Prod. Res., 2011, 13, 879 CrossRef CAS PubMed.
- M. Arai, T. Kawachi, A. Setiawan and M. Kobayashi, ChemMedChem, 2010, 5, 1919 CrossRef CAS PubMed.
- N. Kotoku, S. Fujioka, C. Nakata, M. Yamada, Y. Sumii, T. Kawachi, M. Arai and M. Kobayashi, Tetrahedron, 2011, 67, 6673 CrossRef CAS PubMed.
- W. He, X. Lin, T. Xu, J. H. Jung, H. Yin, B. Yang and Y. Liu, Chem. Nat. Compd., 2012, 48, 208 CrossRef CAS.
- P. Ettinger-Epstein, C. A. Motti, R. De Nys, A. D. Wright, C. N. Battershill and D. M. Tapiolas, J. Nat. Prod., 2007, 70, 648 CrossRef CAS PubMed.
- R. Ueoka, Y. Nakao, S. Fujii, R. W. M. van Soest and S. Matsunaga, J. Nat. Prod., 2008, 71, 1089 CrossRef CAS PubMed.
- E. D. DeSilva and P. J. Scheuer, Tetrahedron Lett., 1980, 21, 1611 CrossRef CAS.
- R. C. Cambie, P. A. Craw, P. R. Bergquist and P. Karuso, J. Nat. Prod., 1988, 51, 331 CrossRef.
- E. D. DeSilva and P. J. Scheuer, Tetrahedron Lett., 1981, 22, 3147 CrossRef CAS.
- M. E. Skindersoe, P. Ettinger-Epstein, T. B. Rasmussen, T. Bjarnsholt, R. De Nys and M. Givskov, Mar. Biotechnol., 2008, 10, 56 CrossRef CAS PubMed.
- A. Gauvin-Bialecki, M. Aknin and J. Smadja, Molecules, 2008, 13, 3184 CrossRef CAS PubMed.
- C. A. Motti, P. Ettinger-Epstein, R. H. Willis and D. M. Tapiolas, Mar. Drugs, 2010, 8, 190 CrossRef CAS PubMed.
- R. Forestier, C. E. Merchant, N. J. De Voogd, T. Matainaho, T. J. Kieffer and R. J. Andersen, Org. Lett., 2009, 11, 5166 CrossRef PubMed.
- M. H. Uddin, M. Otsuka, T. Muroi, A. Ono, N. Hanif, S. Matsuda, T. Higa and J. Tanaka, Chem. Pharm. Bull., 2009, 57, 885 CrossRef CAS.
- M. Hirashima, K. Tsuda, T. Hamada, H. Okamura, T. Furukawa, S. Akiyama, Y. Tajitsu, R. Ikeda, M. Komatsu, M. Doe, Y. Morimoto, M. Shiro, R. W. M. van Soest, K. Takemura and T. Iwagawa, J. Nat. Prod., 2010, 73, 1512 CrossRef CAS PubMed.
- J. Li, L. Du, M. Kelly, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2013, 76, 1492 CrossRef CAS PubMed.
- C. Audoin, D. Bonhomme, J. Ivanisevic, M. de la Cruz, B. Cautain, M. Candida Monteiro, F. Reyes, L. Rios, T. Perez and O. P. Thomas, Mar. Drugs, 2013, 11, 1477 CrossRef CAS PubMed.
- Y. Matsuo, M. Suzuki and M. Masuda, Chem. Lett., 1995, 1043 CrossRef CAS.
- Y. Morimoto, H. Yata and Y. Nishikawa, Angew. Chem., Int. Ed., 2007, 46, 6481 CrossRef CAS PubMed.
- M. Suzuki, Y. Matsuo, S. Takeda and T. Suzuki, Phytochemistry, 1993, 33, 651 CrossRef CAS.
- Y. Morimoto, T. Okita, M. Takaishi and T. Tanaka, Angew. Chem., Int. Ed., 2007, 46, 1132 CrossRef CAS PubMed.
- E. Manzo, M. Gavagnin, G. Bifulco, P. Cimino, S. Di Micco, M. L. Ciavatta, Y. W. Guo and G. Cimino, Tetrahedron, 2007, 63, 9970 CrossRef CAS PubMed.
- J. W. Blunt, M. P. Hartshorn, T. J. McLeannan, M. H. G. Munro, W. T. Robinson and S. C. Yorke, Tetrahedron Lett., 1978, 69 CrossRef CAS.
- Y. Matsuo, M. Suzuki, M. Masuda, T. Iwai and Y. Morimoto, Helv. Chim. Acta, 2008, 91, 1261 CrossRef CAS.
- N.-Y. Ji, X.-M. Li, H. Xie, J. Ding, K. Li, L.-P. Ding and B.-G. Wang, Helv. Chim. Acta, 2008, 91, 1940 CrossRef CAS.
- F. Cen-Pacheco, L. Nordstroem, M. Luisa Souto, M. Norte Martin, J. Javier Fernandez and A. Hernandez Daranas, Mar. Drugs, 2010, 8, 1178 CrossRef CAS PubMed.
- A. R. B. Ola, A.-M. Babey, C. Motti and B. F. Bowden, Aust. J. Chem., 2010, 63, 907 CrossRef CAS.
- B. Vera, A. D. Rodriguez, E. Aviles and Y. Ishikawa, Eur. J. Org. Chem., 2009, 5327 CrossRef CAS PubMed.
- T. Murata, T. Miyase, F. W. Muregi, Y. Naoshima-Ishibashi, K. Umehara, T. Warashina, S. Kanou, G. M. Mkoji, M. Terada and A. Ishih, J. Nat. Prod., 2008, 71, 167 CrossRef CAS PubMed.
- F. Cen-Pacheco, J. A. Villa-Pulgarin, F. Mollinedo, M. Norte, A. H. Daranas and J. J. Fernandez, Eur. J. Med. Chem., 2011, 46, 3302 CrossRef CAS PubMed.
- F. Cen Pacheco, J. A. Villa-Pulgarin, F. Mollinedo, M. Norte Martin, J. Javier Fernandez and A. Hernandez Daranas, Mar. Drugs, 2011, 9, 2220 CrossRef PubMed.
- P. Metzger and M. N. Rager, Tetrahedron Lett., 2002, 43, 2377 CrossRef CAS.
- T. Kajikawa, S. Hasegawa, T. Iwashita, T. Kusumoto, H. Hashimoto, D. M. Niedzwiedzki, H. A. Frank and S. Katsumura, Org. Lett., 2009, 11, 5006 CrossRef CAS PubMed.
- K. H. Shaker, M. Muller, M. A. Ghani, H.-M. Dahse and K. Seifert, Chem. Biodiversity, 2010, 7, 2007 CAS.
- M. Ito, Y. Hirata, Y. Shibata, A. Sato and K. Tsukida, J. Nutr. Sci. Vitaminol., 1987, 33, 313 CrossRef CAS.
- S. S. Chang and R. K. Trench, Proc. R. Soc. B, 1982, 215, 191 CrossRef CAS.
- E. Markifischer and C. H. Eugster, Helv. Chim. Acta, 1988, 71, 24 CrossRef.
- E. Markifischer and C. H. Eugster, Helv. Chim. Acta, 1988, 71, 1689 CrossRef.
- M. Ito, Y. Hirata, K. Tsukida, N. Tanaka, K. Hamada, R. Hino and T. Fujiwara, Chem. Pharm. Bull., 1988, 36, 3328 CrossRef CAS.
- J. Gross and G. Eckhardt, Phytochemistry, 1981, 20, 2267 CrossRef CAS.
- E. Markifischer, R. Buchecker, C. H. Eugster, G. Englert, K. Noack and M. Vecchi, Helv. Chim. Acta, 1982, 65, 2198 CrossRef.
- E. Markifischer, R. Buchecker and C. H. Eugster, Helv. Chim. Acta, 1984, 67, 461 CrossRef.
- M. Acemoglu and C. H. Eugster, Helv. Chim. Acta, 1984, 67, 471 CrossRef CAS.
- W. Eschenmoser, E. Markifischer and C. H. Eugster, Helv. Chim. Acta, 1984, 67, 170 CrossRef CAS.
- L. B. Dealmeida, M. D. C. Penteado, K. L. Simpson, G. Britton, M. Acemoglu and C. H. Eugster, Helv. Chim. Acta, 1986, 69, 1554 CrossRef.
- P. Foss, T. Skjetne and S. Liaaenjensen, Acta Chem. Scand., Ser. B, 1986, 40, 172 CrossRef PubMed.
- T. Kajikawa, K. Aoki, T. Iwashita, D. M. Niedzwiedzki, H. A. Frank and S. Katsumura, Org. Biomol. Chem., 2010, 8, 2513 CAS.
- T. Maoka, N. Akimoto, M.-J. Yim, M. Hosokawa and K. Miyashita, J. Agric. Food Chem., 2008, 56, 12069 CrossRef CAS PubMed.
- V. R. Macherla, J. N. Liu, C. Bellows, S. Teisan, B. Nicholson, K. S. Lam and B. C. M. Potts, J. Nat. Prod., 2005, 68, 780 CrossRef CAS PubMed.
- R. Riclea and J. S. Dickschat, Chem.–Eur. J., 2011, 17, 11930 CrossRef CAS PubMed.
- W. Fenical and P. R. Jensen, Nat. Chem. Biol., 2006, 2, 666 CrossRef CAS PubMed.
- H. C. Kwon, A. P. D. M. Espindola, J.-S. Park, A. Prieto-Davo, M. Rose, P. R. Jensen and W. Fenical, J. Nat. Prod., 2010, 73, 2047 CrossRef CAS PubMed.
- R. Raju, A. M. Piggott, L. X. B. Diaz, Z. Khalil and R. J. Capon, Org. Lett., 2010, 12, 5158 CrossRef CAS PubMed.
- K. Scherlach, J. Schuemann, H.-M. Dahse and C. Hertweck, J. Antibiot., 2010, 63, 375 CrossRef CAS PubMed.
- G. Zhang, S. Sun, T. Zhu, Z. Lin, J. Gu, D. Li and Q. Gu, Phytochemistry, 2011, 72, 1436 CrossRef CAS PubMed.
- W. Balansa, R. Islam, D. F. Gilbert, F. Fontaine, X. Xiao, H. Zhang, A. M. Piggott, J. W. Lynch and R. J. Capon, Bioorg. Med. Chem., 2013, 21, 4420 CrossRef CAS PubMed.
- W. Balansa, R. Islam, F. Fontaine, A. M. Piggott, H. Zhang, T. I. Webb, D. F. Gilbert, J. W. Lynch and R. J. Capon, Bioorg. Med. Chem., 2010, 18, 2912 CrossRef CAS PubMed.
- E. Oldfield and F.-Y. Lin, Angew. Chem., Int. Ed., 2012, 51, 1124 CrossRef CAS PubMed.
- J. Gershenzon and N. Dudareva, Nat. Chem. Biol., 2007, 3, 408 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |