Chemical structure dependent electron transport in 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene
Arunandan Kumar†
*a,
Priyanka Tyagiab,
M. Ananth Reddycd,
G. Malleshamcd,
K. Bhanuprakashde,
V. Jayathirtha Raoce,
M. N. Kamalasanana and
Ritu Srivastavaae
aCenter for Organic Electronics, National Physical Laboratory (Council of Scientific and Industrial Research), Dr. K. S. Krishnan Road, New Delhi-110012, India. E-mail: kumar.arunandan@gmail.com; ritu@mail.nplindia.org
bCenter for Applied Research in Electronics, Indian Institute of Technology Delhi, New Delhi-110016, India
cCrop Protection Chemicals Division, CSIR-Indian Institute of Chemical Technology, Uppal Road, Tarnaka, Hyderabad-500007, India. E-mail: jrao@iict.res.in
dInorganic & Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, Uppal Road, Tarnaka, Hyderabad-500007, India
eNetworking Institute for Solar Energy, New Delhi, India
First published on 13th February 2014
Abstract
In this work, we present a detailed analysis on electron transport studies of 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene (OXD-PH, OXD-PTOL and OXD-OTOL). The effect of methyl substitution at ortho (OXD-OTOL) and para position (OXD-PTOL) on the phenyl ring on the electron transport properties was studied and the results were compared with the anthracene derivative without any substitution at the phenyl ring. Electron transport was found to be highly dependent on the methyl substitution and electron mobilities in OXD-PTOL and OXD-OTOL were found to be lower than in OXD-PH. Mobilities were also found to be different for OXD-PTOL and OXD-OTOL, which indicates that the substitution at different places did not have a similar effect on charge transport properties. Thickness dependent trap states were observed for all three molecules with thickness dependent electron mobilities. Electron mobility was found to increase in all three molecules with the decrease in thickness, which favors their use for organic electronic devices and all three molecules had a better electron transport in comparison to Alq3. These results were explained by the DFT calculation which showed a dihedral structure. The dihedral angle was found to reduce in the anionic form of these molecules. Therefore, these molecules are likely to favor a proper stacking in the solid state form.
Introduction
Organic electronic devices have emerged as strong candidates for future electronic and optoelectronic technology due to the progress made in past two decades.1–5 Several efforts have been made to develop efficient materials and techniques to improve their performances.6–14 The recent advent of the synthesis of semiconducting organic molecules has led to a greater flexibility on the choice of materials in the field of organic electronics.15–19 The combined efforts of theory and experiments have improved the chemical structures and provided efficient organic molecules with unique capabilities.20–24 The organic molecules have different functionalities such as hole transport, emission, electron transport and charge carrier blocking.25–29 In the initial times of research, the devices were based on bilayer structures having one layer for hole transport and other for electron transport and emission.1 The bilayer structures were found to have limitation on maximum achievable efficiencies. Current state of art OLEDs utilizes different layers for different functions such as hole transport, emission and electron transport. Organic molecules are intrinsically found to be p-type and, therefore, the development of efficient electron transporting molecules has always remained a challenge and electron transport materials have less reports in comparison to the hole transport materials.Since the dawn of OLEDs, tris(8-hydroxy quinoline)aluminum (Alq3) has been used as an efficient electron transport material.1 It has good thermal stability with a very high glass transition temperature. However, its electron affinity (EA) is very low, which requires low work function metals or additional layers for the work function modification to form an ohmic contact. Additionally, its conductivity depends on the degree of purity. Similar to Alq3, Bphen has also been widely used as electron transport material due to its high mobility in comparison to Alq3. However, it also has low EA value and, additionally, it has very poor thermal stability with a very low glass transition temperature which is disadvantage in terms of life time. Therefore, several new electron rich molecules containing aromatic heterocyclic derivatives like oxadiazole, triazole, triazine, pyridine etc. have been synthesized and used as electron transport materials.30–39 Molecules containing oxadiazole moieties have been found to possess attracting electron transport properties.40–42 PBD is an oxadiazole molecule, which has been widely used as electron transport material.43 Since it has low EA of 2.16 eV, good electron injection could be achieved only with low work function metal like Ca. Since low work function metals suffer from instability problems, higher work function metals like Al is preferred as cathode in optoelectronic devices. Therefore for good electron injection the EA of the ETL should be high enough. Efforts were made to enhance the EA of PBD by attaching a second oxadiazole group to it. This increases the EA of PBD to 2.8 eV. The attached dimeric oxadiazoles also have an additional advantage of higher glass transition temperature Tg.44 Further replacing the central phenylene moiety in PBD, which lies in between the two oxadiazoles, by ethylene and by pyridyl, the EA of the molecule could be further increased. At high electric fields electron mobilities of 2 × 10−5 cm2 V−1 s−1 has been reported for PBD and related compounds.
Anthracene is a molecule that has been widely investigated as an emitting material for OLEDs. The substitution of acceptor or donor at 2 and 6 positions of anthracene gave rise to n- and p-type activity.45,46 In the present work the central phenylene moiety in PBD has been replaced with anthracene to enhance its emission properties so as to have good electron transporting property due to the attached oxadiazole groups. Many anthracene derivatives with various electron donating groups and withdrawing groups have been synthesized and characterized as emissive layers.47–51 However, their electron transporting properties remained still less understood and requires detailed studies. In the present work, we have focused on the electron transporting properties of 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene and studied the effect of substitution of a methyl group at phenyl ring.
Experimental
The electron transport study was performed on the electron only devices which were fabricated onto glass substrate, which were cleaned sequentially using deionized water, acetone, trichloroethylene and isopropyl alcohol for 20 min each using an ultrasonic bath and dried in vacuum. Devices were fabricated in sandwich structure, the vacuum evaporated organic layer sandwiched between two vacuum evaporated aluminum electrodes. The size of the pixels was 4 × 4 mm2. The organic material was deposited at the rate of 0.1 nm s−1 while Al was evaporated at 0.5 nm s−1. The thickness of Al electrode was 150 nm and the whole deposition sequence were performed without breaking the vacuum. Thickness of deposited films was measured using a quartz crystal thickness monitor. The J–V characteristics of the devices were measured under vacuum using a Keithley 2400 source measure unit and a homemade liquid nitrogen cryostat. The injection property of organic material on metal and metal on organic were also checked by reversing the biasing and were found to be similar and within experimental error. The thickness of organic layers was reconfirmed by ellipsometric methods. The active area was measured by using a traveling microscope.Results and discussion
In this work, we have studied the effect of substituent on charge transporting properties of 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene. Three molecules were synthesized with the synthesis procedure published earlier15 and their chemical structures are shown in Fig. 1. The difference between these molecules was in the substitution at the phenyl ring. The first molecule has no substitution, second has a methyl group at the para position and third molecule at ortho position. These molecules were having an anthracene backbone and were modified by 1,3,4-oxadiazole to increase their electron transporting properties. These molecules were found to possess good thermal stability having a decomposition temperature of 461, 400 and 445 °C for OXD-PH, OXD-PTOL and OXD-OTOL, respectively. Further, glass transition temperature is an important parameter and it should be sufficiently high for the organic molecules in order to have their optimum utilization in organic electronic devices. Their glass transition temperatures were measured and were found to be 97, 106 and 83 °C. These glass transition temperatures are high enough for their utilization in OLEDs. For a molecule to be used as an efficient electron transport material, it should possess very high electron affinity so that it can form an ohmic contact with stable metal electrodes such as Al. Electrochemical characterization of these molecules were performed and their electron affinity were found to be close to 3.2 eV. This value is almost 0.5 eV higher than the values for commonly used electron transport materials such as Alq3 and Bphen, which have electron affinity close to 2.7 eV. This motivated us to carry out the electron transporting properties of these molecules and to observe the change in electron transport by the substitution in the first molecule at phenyl ring.15Electron transport studies were performed by fabricating electron only devices of these molecules with a device structure of Al (150 nm)/ETM/Al (150 nm), where the thickness of ETM was varied. Aluminum has a work function of about 3.8 eV, the EA values for these molecules were 3.2 eV and the IP values were more than 6.0 eV. Thereby, the barrier for electron injection (∼0.6 eV) was very low in comparison to the barrier for hole injection (>2.2 eV). Therefore, these devices may be considered as electron only devices. We have fabricated electron only devices for all three molecules and measured the current density–voltage (J–V) characteristics at ambient conditions. Fig. 2 shows the J–V characteristics in double log scale for the electron only devices fabricated using 80 nm thicknesses of OXD-PH, OXD-PTOL and OXD-OTOL. It can be seen from this figure that OXD-PH has highest current density value and OXD-PTOL has the lowest. Since, all of these molecules were having similar EA values, thereby, having the same electron injection barrier, the change in J–V characteristics can be solely considered due to the change in conductivity of the molecule. This indicates that the methyl substitution at phenyl ring caused a decrease conductivity of these molecules and this substitution has a greater effect at para position in comparison to that at ortho position.
 | ||
| Fig. 2 J–V characteristics in double log scale of the electron only devices with OXD-PH, OXD-PTOL and OXD-OTOL as an active layer having 80 nm thicknesses. | ||
J–V characteristics were found to be ohmic at lower values of bias voltages (V < 1 V). This may attributed as due to the background doping by the impurities. As the voltage has increased the current density started to increase as a nonlinear function of voltage (J ∝ Vm, with m > 2). This indicates towards trap charge limited conduction (TCLC) mechanism in our devices, in which the current density is given as52
 | (1) |
 | ||
| Fig. 3 J–V characteristics for electron only devices of (a) OXD-PH, (b) OXD-PTOL and (c) OXD-OTOL at different temperatures. | ||
To investigate the conduction mechanism in detail, we have selected the electron only device of OXD-PH and analyzed the J–V characteristics using eqn (1). In TCLC mechanism, current density also scales with some power of inverse of the thickness. For the confirmation of the conduction mechanism, thickness dependent J–V measurements were also performed. Electron only devices were fabricated having 75, 80, 100 and 120 nm thicknesses of OXD-PH. Fig. 4 shows the J–V characteristics of electron only devices with different thicknesses of OXD-PH. The inset of this figure shows the current density as a function of 1/d2l+1 at 7, 8 and 9 V; and it can be seen that current density scaled nearly linearly with d2l+1. A small variation from the linearity is due to the thickness dependent trap energy and trap density values. This confirmed the conduction mechanism is TCLC. Thickness dependent J–V measurements were also performed for the electron only devices with other two molecules and the conduction mechanism was confirmed as TCLC mechanism in those molecules.
 | ||
| Fig. 4 J–V characteristics of electron only devices of OXD-PH having 75, 80, 100 and 120 nm thicknesses. The inset of figure shows the thickness scaling (1/d2l+1) of current density at 7, 8 and 9 V. | ||
In TCLC mechanism, J ∝ Vl+1, where l = Tc/T, Tc is a constant and depends on trap energy (Et = kTc). The value of l was obtained from the J–V characteristics of OXD-PH at each temperature and plotted as a function of 1000/T in Fig. 5 for the devices of different thicknesses. The value of trap energy was calculated using the slope of l vs. 1000/T plot for all thicknesses and were found to be 185, 200, 230 and 280 meV for the devices with 75, 80, 100 and 120 nm thicknesses of OXD-PH, respectively. Therefore, the trap energy was found to be highly dependent of thickness of OXD-PH. Similarly, we have also obtained the value of trap energy for other two molecules OXD-PTOL and OXD-OTOL for different thicknesses. The values of trap energy were found to be 78, 93 and 130 meV for the devices with 80, 100 and 120 nm thicknesses of OXD-PTOL. These values were found to be 98, 125 and 160 meV for the devices with 80, 100 and 120 nm thicknesses of OXD-OTOL. The thickness dependent trap energy data indicate that the trap energy is highly dependent on thickness for the devices with each material and the substitution has also changed the trap energy drastically in these molecules. OXD-PH, the molecule without substitution, has a very high trap energy value, in comparison to the molecules with substitution. This indicates that the substitution has reduced the energy of trap depth and this reduction is affected by the position of substitution. The molecule with substitution at ortho position has the lowest trap energy, which means that in this molecule the trap levels are very near to the transport levels. Therefore, the trap levels can be filled in this molecule with the increase of operating electric field and the electron only devices may show a trap filled space charge limited conduction regime. In this regime current density is proportional to the square of voltage. It can be seen from Fig. 3(c) that after V = 7 V, the slope of J–V characteristics has changed and found to be very close to 2. Therefore, this suggests that the electron only devices with OXD-PTOL have three conduction regimes in their J–V characteristics. Similarly, the devices with OXD-OTOL has also found to possess trap filled space charge limited conduction regime for 80 nm thickness only and this region was not observed for higher thicknesses. However, this region was completely found missing in the devices with OXD-PH. The reason behind the missing trap filling region could be the very high value of trap energy and the traps were not been able to be filled by increasing the field in our operating regimes. Further, for quantifying the thickness dependence of trap energy, change in trap energy per unit increase in thickness of the active layer was calculated. These values were found to be 2.11, 1.3 and 1.55 meV nm−1 for the electron only devices with OXD-PH, OXD-PTOL and OXD-OTOL, respectively. Therefore, the thickness dependence of trap energy was highest for OXD-PH and lowest for OXD-PTOL. This suggests that the thickness dependence of current density should also vary for the devices with these molecules. As discussed earlier, the current density was found to scale with 1/d2l+1 for the devices with all three molecules. However, the value of l was found to be different for each molecule for same temperature. This value was found to be highest for the devices with OXD-PH and lowest for the devices with OXD-PTOL for all temperatures. Therefore, thickness dependence of current density was found to be highest for the devices with OXD-PH and lowest for the devices with OXD-PTOL. It is worth noting that the trap energy has decreased with the decrease in thickness for each molecule.
 | ||
| Fig. 5 Slope of power law dependent current density (l) as a function of 1000/T of electron only devices with 75, 80, 100 and 120 nm thicknesses of OXD-PH. | ||
Further, the devices with OXD-PTOL were found to possess trap filled space charge limited conduction regime after a certain voltage. This voltage at which traps start to fill, is known as trap filling voltage (VTFL) and given by52
 | (2) |
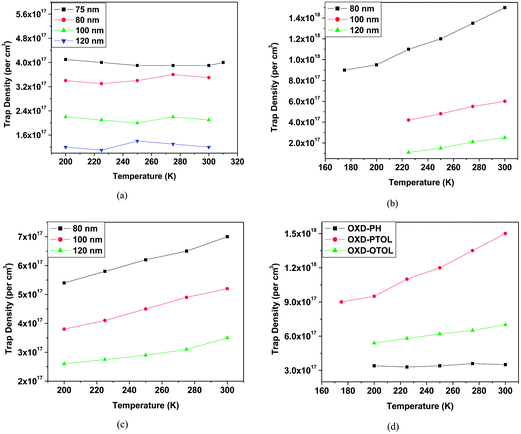
The value of trap density is required, in addition to, the trap energy for a complete analysis of trap states. Eqn (2) was utilized to calculate the values of trap density for OXD-PTOL and for one thickness of OXD-OTOL at all temperatures and for the other data eqn (1) was used to estimate this value. Fig. 6 shows the plot of trap energy and trap density as a function of thicknesses for OXD-PH, OXD-PTOL and OXD-OTOL. It can be seen from the figure that the trap density has increased with the decrease in trap energy. This observation is in complete agreement with the traps having an exponential type of distribution, in which, trap density is given as52
 | (3) |
Trap density was found to decrease with the decrease in thickness. OXD-PTOL has the largest variation of trap density with the thickness and OXD-PH has the smallest. Trap density has varied from 1.5 × 1018 per cm3 to 2.5 × 1017 per cm3 corresponding to a thickness variation of 80 nm to 120 nm of OXD-PTOL. The value of trap density was found to be the lowest for OXD-PH, which was 4 × 1017 per cm3 for 75 nm thickness of OXD-PH and has decreased to 1.2 × 1017 per cm3 for 120 nm thickness of OXD-PH. OXD-OTOL has found to possess an intermediate trap density values to OXD-PTOL and OXD-PH, having the values of 7 × 1017 per cm3 to 3.5 × 1017 for 80 and 120 nm thicknesses, respectively. The data of trap energy and trap density show that the trap energy and trap density were highly dependent on thickness for these materials. This thickness dependence may be due to the change in morphology for these materials with the change in thickness. Fig. 7 shows the AFM images of 80, 100 and 120 nm thicknesses of OXD-PTOL and the roughness values calculated for these thicknesses from AFM images were found to be 1.3, 1.5 and 1.8 nm for 80, 100 and 120 nm thicknesses respectively. This shows that the morphology has changes with the change in thickness which may be the reason for the change in trap energy and density.
Fig. 8 shows the value of trap density as a function of temperature for these materials. It can be seen from the figure that trap density was found to be highly temperature dependent (decreased with the increase in temperature) for 80 nm thickness of OXD-PTOL and this temperature dependence has decreased with the increase in thickness, however, trap density is temperature dependent for all thicknesses of OXD-PTOL. Trap density has shown a little temperature dependence for 80 nm thickness of OXD-OTOL and this temperature dependence disappeared for higher thicknesses of OXD-OTOL. Trap density was completely found temperature independent for OXD-PH. It can be observed from this temperature dependence trap density data that the devices, for which the trap filled SCLC was observed, have temperature dependence trap density. This may be ascribed by considering the low value of trap energy. As temperature increases, some of the trap states started to fill and filled trap states do not contribute in charge transport. Therefore, they do not affect the charge carrier mobility and practically do not work as trap states, thereby, reducing the trap density. The devices, which has higher value of trap energy, has very minor affect due to the high lying trap states, even at very low temperatures and therefore, filling of these trap states at high temperature do not affect the charge carrier mobility and charge carrier transport. Therefore, trap densities has not decreased for such devices with the increase in temperature.
Presence of trap states affects the charge carrier mobility in space charge limited conduction. Therefore, charge carrier mobilities have been calculated for each device to have a further, in depth analysis, of the charge carrier transport mechanism. In organic semiconductors, the charge carrier mobility is generally field dependent and this dependence has a PF type exponential form 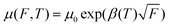 , where parameters μ0 and β are zero field mobility and field dependent factor, respectively. J–V characteristics were fitted using eqn (1) with a field dependent mobility by varying the values of μ0 and β. Fig. 9 shows the experimental and fitted J–V characteristics for electron only devices with 80 nm thicknesses of OXD-PH, OXD-PTOL and OXD-OTOL at 300 K temperature and fitted J–V characteristic resembled very closely to the experimental J–V characteristics. Similarly, we have fitted the J–V characteristics for all devices by varying μ0 and β. Fig. 10–12 show the fitted values of μ0 and β as a function of temperature for the electron only devices with OXD-PH, OXD-PTOL and OXD-OTOL. Zero field mobility was found to decrease with the decrease in temperature and field dependence was found to increase with the decrease in temperature. The magnitude of zero field mobility has decreased by orders of magnitude by decreasing the temperature from 300 K to 200 K. The field dependency has increased by two or three times with the same change in temperature. The most worth noting point from Fig. 10–12 is the thickness dependence of mobility. OXD-PH has the highest change in charge carrier mobility with thickness and OXD-PTOL has the lowest. Generally, zero field mobilities are thickness independent in organic semiconductors. Presence of trap states decreases the zero field mobility and this decrease in dependent on trap energy and trap density. It can be observed from Fig. 10–12 that charge carrier mobility has decreased with the increase in thickness for all three materials. It indicates that as the trap energy has increased with the increase in thickness, charge carrier mobility has decreased. Highest change in trap energy was observed for OXD-PH and the lowest for OXD-PTOL. Therefore, the maximum change in mobility was observed for OXD-PH and the minimum for OXD-PTOL. Field dependent factor has increased with the increase in temperature for all the devices and this factor have not varied by a large amount such as zero field mobility. The common observation for these molecules was the charge carrier mobility has increased with the decrease in thickness. In application of OLEDs, the commonly used thicknesses of ETLs are 30–45 nm and high mobilities of these molecules for lower thicknesses favors their use as ETLs in OLEDs.53,54 Further, we have calculated the charge carrier mobilities for these molecules using the zero field mobility and field dependent factor at different field values. Fig. 13 shows the charge carrier mobilities for these three molecules at different electric field values for 80 nm thick active layers. It can be seen from this figure that the charge carrier mobilities can reach the value as high as 10−4 cm2 V−1 s−1 for the electric field values of interest for OLEDs (∼1 × 106 V cm−1). And these values are almost one order of magnitude higher than the corresponding values for Alq3.
, where parameters μ0 and β are zero field mobility and field dependent factor, respectively. J–V characteristics were fitted using eqn (1) with a field dependent mobility by varying the values of μ0 and β. Fig. 9 shows the experimental and fitted J–V characteristics for electron only devices with 80 nm thicknesses of OXD-PH, OXD-PTOL and OXD-OTOL at 300 K temperature and fitted J–V characteristic resembled very closely to the experimental J–V characteristics. Similarly, we have fitted the J–V characteristics for all devices by varying μ0 and β. Fig. 10–12 show the fitted values of μ0 and β as a function of temperature for the electron only devices with OXD-PH, OXD-PTOL and OXD-OTOL. Zero field mobility was found to decrease with the decrease in temperature and field dependence was found to increase with the decrease in temperature. The magnitude of zero field mobility has decreased by orders of magnitude by decreasing the temperature from 300 K to 200 K. The field dependency has increased by two or three times with the same change in temperature. The most worth noting point from Fig. 10–12 is the thickness dependence of mobility. OXD-PH has the highest change in charge carrier mobility with thickness and OXD-PTOL has the lowest. Generally, zero field mobilities are thickness independent in organic semiconductors. Presence of trap states decreases the zero field mobility and this decrease in dependent on trap energy and trap density. It can be observed from Fig. 10–12 that charge carrier mobility has decreased with the increase in thickness for all three materials. It indicates that as the trap energy has increased with the increase in thickness, charge carrier mobility has decreased. Highest change in trap energy was observed for OXD-PH and the lowest for OXD-PTOL. Therefore, the maximum change in mobility was observed for OXD-PH and the minimum for OXD-PTOL. Field dependent factor has increased with the increase in temperature for all the devices and this factor have not varied by a large amount such as zero field mobility. The common observation for these molecules was the charge carrier mobility has increased with the decrease in thickness. In application of OLEDs, the commonly used thicknesses of ETLs are 30–45 nm and high mobilities of these molecules for lower thicknesses favors their use as ETLs in OLEDs.53,54 Further, we have calculated the charge carrier mobilities for these molecules using the zero field mobility and field dependent factor at different field values. Fig. 13 shows the charge carrier mobilities for these three molecules at different electric field values for 80 nm thick active layers. It can be seen from this figure that the charge carrier mobilities can reach the value as high as 10−4 cm2 V−1 s−1 for the electric field values of interest for OLEDs (∼1 × 106 V cm−1). And these values are almost one order of magnitude higher than the corresponding values for Alq3.
 | ||
| Fig. 9 Experimental and fitted J–V characteristics for electron only devices with 80 nm thicknesses of OXD-PH, OXD-PTOL and OXD-OTOL. | ||
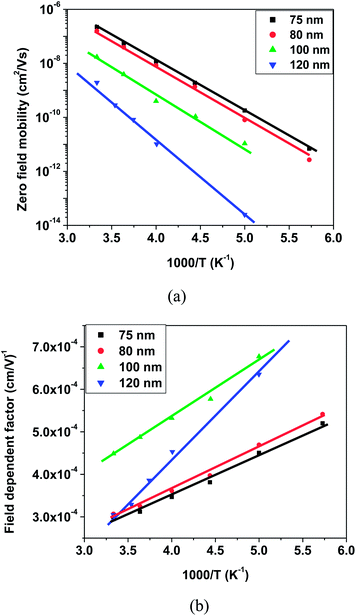 | ||
| Fig. 10 Fitted values of (a) zero field mobilities and (b) field dependent factor as a function of temperature for electron only devices with OXD-PH. | ||
 | ||
| Fig. 11 Fitted values of (a) zero field mobilities and (b) field dependent factor as a function of temperature for electron only devices with OXD-PTOL. | ||
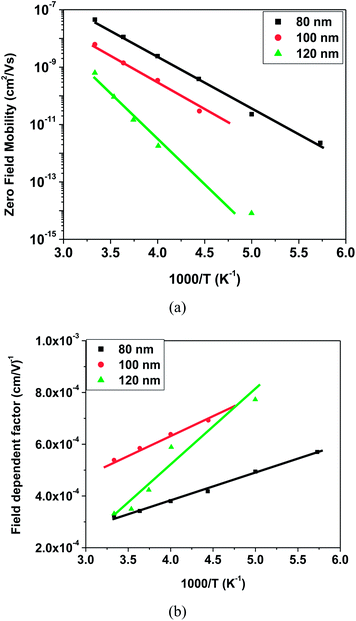 | ||
| Fig. 12 Fitted values of (a) zero field mobilities and (b) field dependent factor as a function of temperature for electron only devices with OXD-OTOL. | ||
 | ||
| Fig. 13 Mobility values for OXD-PH, OXD-PTOL and OXD-OTOL for the electron only devices with 80 nm thicknesses of active layer at different electric field values. | ||
OLEDs with a device structure ITO (120 nm)/α-NPD (30 nm)/5% Ir(ppy)3 doped CBP (35 nm)/BCP (6 nm)/ETL (28 nm)/LiF (1 nm)/Al (150 nm). N,N′-Di-[(1-naphthalenyl)-N,N′-diphenyl]-(1-1′-biphenyl)-4,4′-diamine (α-NPD) (Sigma Aldrich) was used as the hole transporting layers. 2,9-Dimethyl-4,7-diphenyl-1,10 phenanthrolene (BCP) (Sigma Aldrich) which has a high ionization potential (6.5 eV) has been used as hole blocking layer and lithium fluoride (LiF)/aluminium (Al) and ITO has been used as cathode and anode respectively. Devices with OXD-PH, OXD-PTOL and OXD-OTOL as ETLs were fabricated and for comparison we have also fabricated OLED with Alq3 as ETL. All the OLEDs with OXD molecules as ETL have higher luminous intensities in comparison to the OLED with Alq3 as an ETL. OLED with OXD-PH as ETL has the highest luminous intensity and OXD-PTOL has the lowest. Fig. 14 shows the I–V–L characteristics for these OLEDs with different electron transport layers. It can be seen from the figure that the OLEDs with oxadiazole molecules possess superior performance in comparison to the OLED with Alq3 as ETL. The device with OXD-PH as ETL was found to be the most efficient. Luminous intensities were found to be 1000, 1200, 1400 and 1600 Cd m−2 at 8 V for the OLEDs with Alq3, OXD-PTOL, OXD-OTOL and OXD-PH as ETL, respectively.
 | ||
| Fig. 14 Current–voltage–luminous intensity characteristics for OLEDs with Alq3, OXD-PH, OXD-PTOL and OXD-OTOL as ETLs. | ||
Our electron transport studies have shown that the oxadiazole molecules possess higher electron transporting properties in comparison to the well established ETL Alq3. Further, substitution of a methyl group at the phenyl ring has a stronger effect on electron transporting properties without having any significant effect on the molecular orbital and emission properties. Our theoretical calculation suggested that the substitution at phenyl ring has changed the bond lengths, angles and dihedral angles. All three molecules were found to possess a dihedral shape with significant values of dihedral angles of nearly 51–52°.15 We have also listed these values in Table 1. These angles reduce significantly by about 15° for anionic form of the molecules and the molecular geometry has tended towards a more planar one. In organic semiconducting molecules, charge carrier transport is dependent on the intermolecular stacking and molecules having a planar geometry are more likely to form properly stacked layers. Most of the hole transporting molecules possess planar molecular geometry, thereby, having proper stacking in their solid state form. Molecules having a geometry composed of more than one molecular plane are more likely to favor the presence of intrinsic trap states in their solid state form. Most of the electron transporting materials have non-planar geometry such as Alq3 possess a trihedral molecular geometry, therefore, the electron transport in Alq3 was found highly affected by the trap states. Trap states reduce the charge carrier mobility and therefore, efficient electron transporting molecules require nearly planar molecular geometry. The studied oxadiazole molecules were found to possess dihedral molecular geometry and the dihedral angle was found to reduce for their anionic form, making the geometry more close to planar. Therefore, these oxadiazole molecules could be strong candidate as electron transporting materials in organic electronic devices. Additional, they have excellent thermal stability high lying EA, making them more useful. Substitution at these molecules may be used to tailor their electron transporting properties without changing their emission characteristics. This provides flexibility to their use as electron transporting materials.
| HOMO (eV) | LUMO (eV) | Dihedral angle | |
|---|---|---|---|
| OXD-PH | −5.92 | −3.19 | −51.957 |
| OXD-PTOL | −6.00 | −3.16 | −52.214 |
| OXD-OTOL | −6.02 | −3.11 | −52.248 |
Conclusion
In conclusion, we have performed electron transport studies on 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene. Effect of methyl substitution at ortho and para position on the phenyl ring was studied. The studies were performed on three molecules OXD-PH (without methyl substitution), OXD-PTOL (methyl substitution at para position) and OXD-OTOL (methyl substitution at ortho position). It was observed that the methyl substitution has changed the current density of the electron transport devices significantly. Devices with OXD-PTOL and OXD-OTOL were found to possess a lower current density in comparison to the device with OXD-PH as active material. Current densities were also found to be different for the devices with OXD-PTOL and OXD-OTOL, which indicates that the substitution at different places did not have a similar effect on charge transport properties. Thickness dependent trap states were observed for all three molecules and their electron mobilities were also found to be thickness dependent. OXD-PH was found to possess highest mobility, while OXD-PTOL had the lowest. Electron mobility was found to increase in all three molecules with the decrease in thickness, which favors their use for organic electronic devices. All three molecules were found to have better electron transport in comparison to the well established electron transport material Alq3.Acknowledgements
The Authors gratefully recognize the financial support from the project NWP-0055 (TAPSUN). One of the author (PT) acknowledges Council of Scientific and Industrial Research for providing the Senior Research Fellowship.References
- C. W. Tang and S. A. Vanslyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS PubMed.
- B. C. Krummacher, V. E. Choong, M. K. Mathai, S. A. Choulis and F. So, Appl. Phys. Lett., 2006, 88, 113506 CrossRef PubMed.
- J. H. Seo, J. H. Park, U. K. Kim, J. W. Hyung, K. H. Lee and S. S. Yoon, Appl. Phys. Lett., 2007, 90, 203507 CrossRef PubMed.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burn and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS.
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem and K. Leo, Nature, 2009, 459, 234 CrossRef CAS PubMed.
- P. Tyagi, R. Srivastava, A. Kumar, G. Chauhan, A. Kumar, S. S. Bawa and M. N. Kamalasanan, Synth. Met., 2010, 160, 1126 CrossRef CAS PubMed.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048 CrossRef CAS PubMed.
- A. Kumar, R. Srivastava, D. S. Mehta and M. N. Kamalasanan, Org. Electron., 2012, 13, 1750 CrossRef CAS PubMed.
- X. Qi and S. R. Forrest, J. Appl. Phys., 2011, 110, 124516 CrossRef PubMed.
- A. Kumar, R. Srivastava, P. Tyagi, D. S. Mehta and M. N. Kamalasanan, Org. Electron., 2012, 13, 159 CrossRef CAS PubMed.
- T. N. Ng, W. R. Silveira and J. A. Marohn, Phys. Rev. Lett., 2007, 98, 066101 CrossRef.
- P. Tyagi, A. Kumar, L. I. Giri, M. K. Dalai, S. Tuli, M. N. Kamalasanan and R. Srivastava, Opt. Lett., 2013, 38, 3854 CrossRef CAS PubMed.
- A. Kumar, P. Tyagi, R. Srivastava, D. S. Mehta and M. N. Kamalasanan, Appl. Phys. Lett., 2013, 102, 203304 CrossRef PubMed.
- K. R. Choudhury, J. H. Yoon and F. So, Adv. Mater., 2008, 20, 1456 CrossRef.
- M. A. Reddy, A. Thomas, K. Srinivas, V. J. Rao, K. Bhanuprakash, B. Sridhar, A. Kumar, M. N. Kamalasanan and R. Srivastava, J. Mater. Chem., 2009, 19, 6172 RSC.
- V. Coropceanu, J. Cornil, D. A. da Silva Filho, Y. Olivier, R. Silbey and J. L. Bredas, Chem. Rev., 2007, 107, 926 CrossRef CAS PubMed.
- E. F. Valeev, V. Coropceanu, D. A. da Silva Filho, S. Salman and J. L. Bredas, J. Am. Chem. Soc., 2006, 128, 9882 CrossRef CAS PubMed.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953 CrossRef CAS PubMed.
- Z. Li and H. Meng, Organic Light-Emitting Materials and Devices, Taylor & Francis Group, LLC, Boca Raton, 2007 Search PubMed.
- E. Ahmed, T. Earmme and S. A. Jenekhe, Adv. Funct. Mater., 2011, 21, 3889 CrossRef CAS.
- D. H. Park, M. S. Kim and J. Joo, Chem. Soc. Rev., 2010, 39, 2439 RSC.
- J. Wang and D. Zhang, Adv. Polym. Technol., 2013, 32, E323 CrossRef CAS.
- D.-J. Liawa, K.-L. Wang, Y.-C. Huang, K.-R. Lee, J.-Y. Lai and C.-S. Ha, Prog. Polym. Sci., 2012, 37, 907 CrossRef PubMed.
- M.-X. Wang, Acc. Chem. Res., 2012, 45, 182 CrossRef CAS PubMed.
- S. Naka, H. Okata, H. Onnagawa, Y. Yamaguchi and T. Tsutsui, Synth. Met., 2000, 111, 331 CrossRef.
- G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC.
- Y.-H. Kim, H.-C. Jeong, S.-H. Kim, K. Yang and S.-K. Kwon, Adv. Funct. Mater., 2005, 15, 1799 CrossRef CAS.
- S. W. Culligan, A. C. A. Chen, J. U. Wallace, K. P. Klubek, C. W. Tang and S. H. Chen, Adv. Funct. Mater., 2006, 16, 1481 CrossRef CAS.
- P. Tyagi, R. Srivastava, A. Kumar, V. K. Rai, R. Grover and M. N. Kamalasanan, Synth. Met., 2010, 160, 756 CrossRef CAS PubMed.
- M. A. Reddy, G. Mallesham, A. Thomas, K. Srinivas, V. J. Rao, K. Bhanuprakasha, L. Giribabu, R. Grover, A. Kumar, M. N. Kamalasanan and R. Srivastava, Synth. Met., 2011, 161, 869 CrossRef CAS PubMed.
- S. Yoo, H. Yun, I. Kang, K. Thangaraju, S. Kwon and Y. Kim, J. Mater. Chem. C, 2013, 1, 2217 RSC.
- M. Ichikawa, K. Wakabayashi, S. Hayashi, N. Yokoyama, T. Koyama and Y. Taniguchi, Org. Electron., 2010, 11, 1966 CrossRef CAS PubMed.
- M. Ichikawa, S. Mochizuki, H.-G. Jeon, S. Hayashi, N. Yokoyama and Y. Taniguchi, J. Mater. Chem., 2011, 21, 11791 RSC.
- H.-W. Lin, C.-W. Lu, L.-Y. Lin, Y.-H. Chen, W.-C. Lin, K.-T. Wong and F. Lin, J. Mater. Chem. A, 2013, 1, 1770 CAS.
- C. Sun, Z. M. Hudson, M. G. Helander, Z.-H. Lu and S. Wang, Organometallics, 2011, 30, 5552 CrossRef CAS.
- W. White, Z. M. Hudson, X. Feng, S. Han, Z.-H. Lu and S. Wang, Dalton Trans., 2010, 39, 892 RSC.
- C. Wang, L. O. Palsson, A. S. Batsanov and M. R. Bryce, J. Am. Chem. Soc., 2006, 128, 3789 CrossRef CAS PubMed.
- Y. Tao, Q. Wang, Y. Shang, C. Yang, L. Ao, J. Qin, D. Ma and Z. Shuai, Chem. Commun., 2009, 79 Search PubMed.
- H. S. Wang, M. S. Su and K. H. Wei, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3331 CrossRef CAS.
- A. P. Kulkarni, C. J. Tonzola, A. Babel and S. A. Jenekhe, Chem. Mater., 2004, 16, 4556 CrossRef CAS.
- G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC.
- S. Oyston, C. Wang, I. F. Perepichka, A. S. Batsanov, M. R. Bryce, J. H. Ahn and M. C. Petty, J. Mater. Chem., 2005, 15, 5164 RSC.
- J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bassler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551 CrossRef CAS.
- C. Wang, G.-Y. Jung, Y. Hua, C. Pearson, M. R. Bryce, M. C. Petty, A. S. Batsanov, A. E. Goeta and J. A. K. Howard, Chem. Mater., 2001, 13, 1167 CrossRef CAS.
- Y.-H. Kim, H.-C. Jeong, S.-H. Kim, K. Yang and S.-K. Kwon, Adv. Funct. Mater., 2005, 15, 1799 CrossRef CAS.
- S. W. Culligan, A. C. A. Chen, J. U. Wallace, K. P. Klubek, C. W. Tang and S. H. Chen, Adv. Funct. Mater., 2006, 16, 1481 CrossRef CAS.
- P.-I. Shih, C.-Y. Chuang, C.-H. Chien, E. W.-G. Diau and C.-F. Shu, Adv. Funct. Mater., 2007, 17, 3141 CrossRef CAS.
- M.-X. Yu, J.-P. Duan, C.-H. Lin, C.-H. Cheng and Y.-T. Tao, Chem. Mater., 2002, 14, 3958 CrossRef CAS.
- W. J. Shen, R. Dodda, C. C. Wu, F. I. Wu, T. H. Liu, H.-H. Chen, C. H. Chen and C.-F. Shu, Chem. Mater., 2004, 16, 930 CrossRef CAS.
- S. Ando, J. I. Nishida, E. Fujiwara, H. Tada, Y. Inoue, S. Tokito and Y. Yamashita, Chem. Mater., 2005, 17, 1261 CrossRef CAS.
- G. Mallesham, S. Balaiah, M. A. Reddy, B. Sridhar, P. Singh, R. Srivastava, K. Bhanuprakash and V. J. Rao, Photochem. Photobiol. Sci., 2013, 14, 342 Search PubMed.
- A. Kumar, R. Srivastava, P. Tyagi, D. S. Mehta, M. N. Kamalasanan, A. Reddy and K. Bhanuprakash, Synth. Met., 2010, 160, 774 CrossRef CAS PubMed.
- P. Tyagi, R. Srivastava, A. Kumar, S. Tuli and M. N. Kamalasanan, Org. Electron., 2013, 14, 1391 CrossRef CAS PubMed.
- A. Kumar, R. Srivastava, P. Tyagi, D. S. Mehta and M. N. Kamalasanan, J. Appl. Phys., 2011, 109, 114511 CrossRef PubMed.
Footnote |
| † Present address: Laboratoire Interdisciplinaire Carnot de Bourgogne (ICB), UMR 6303 CNRS, Universit'e de Bourgogne, 9, Av. Savary, BP 47870, 21078 Dijon Cedex, France. |
| This journal is © The Royal Society of Chemistry 2014 |