Cellulose generated-microporous carbon nanosheets with nitrogen doping†
Wenzhong Shen*a,
Tuoping Huab and
Weibin Fan*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi 030001, P.R. China. E-mail: shenwzh2000@yahoo.com; Fax: +86-351-4041153
bNorthern University of China, Taiyuan, Shanxi 030051, P.R. China
First published on 22nd January 2014
Abstract
Nanosheet porous carbon with surface area of 1854 m2 g−1 and pore volume of 0.82 cm3 g−1 is synthesized using cellulose and KOH–urea, the sheet structure is formed during drying and it was kept after carbonization. The nitrogen-containing groups are incorporated into the carbon matrix through urea decomposition and mainly existed as pyridine-like, pyrrole-like and graphite-like nitrogen species. Unique compositional and structural features endow the nitrogen-doped porous carbon nanosheets with superior CO2 adsorption performance of 5.8 mmol g−1.
Introduction
Porous carbon materials are receiving increasing attention because of their potential applications in highly efficient adsorbents,1 catalysts,2 and energy materials.3 They are currently fabricated by carbonization of various carbon precursors or carbon matrix combining with activation or templating technology.4 The activation using chemicals is to promote the pore developing, while templating is to form pores and control the pore shape.In addition to the commonly concerned porous feature and surface area, the morphology of porous carbons has also attracted great interest in recent years. Several special morphology endowed porous carbon excellent properties. For example, carbon nanotubes confined metal particles with unique properties in chemical reactions;5 carbon nanotube with extremely high tensile strength was controlled wall thick by CVD connected with catalyst;6 cubic ordered mesostructured carbide-derived carbons offered excellent hydrocarbon storage capacitance and good performance as electrode material for supercapacitors;7 shell–core structure porous carbon displaying power density of 11.7 kW kg−1 was obtained using spherical silica as template8 or controlling phase transferring;9 nanosheet graphene having excellent reversible capacity of 1040 mA h g−1 at 100 mA g−1 was synthesized by controlled low-concentration monomicelle close-packing assembly approach.10
Heteroatoms like N and B introduced into porous carbon matrix greatly enhanced electronic chemistry performance due to pseudocapacitive effect,11 increased adsorption properties for polar molecules because of its basic surface groups,12 promoted dispersion of catalyst particle and enhanced the interaction of support and catalyst particle,13 etc.
Recently, some novel synthesis routes to control the morphology of porous carbons were reported, tunable morphologies from micro- to mesoporous with apparent specific surface areas up to 2000 m2 g−1 was reported by templating salt.14 Layered porous carbon composed of nano-graphenes was synthesized by using aminoclay as a template.15 Ultralight carbon nanofiber aerogel with a density of 4–6 mg cm−3 was derived from bacterial cellulose.16 Carbon microtube material was synthesized using ZnO as template by CVD process,17 carbon-based microporous nanoplates containing numerous heteroatoms were fabricated from regenerated silk fibroin,18 nanosheets of g-C3N4 was obtained through thermal oxidation etching its parent bulk material.19
Nanosheet materials, such as, MnO2, TiO2, graphene, have been recognized as a novel class of nanostructured materials due to their unique structural feature of ultimate two-dimensional anisotropy with extremely small thickness in nanometer and even subnanometer scale.20 This characteristic often leads to new physicochemical properties due to the quantum confinement effect.
Hierarchical porous carbon from cotton was prepared in presence of NaOH and urea mixtures,21 and it displayed better properties as catalyst support and electrochemical performances. However, the process needs freeze-dried, and higher ratio of NaOH to cotton, the pore size distribution ranged from micropore to macropore, and the resultant porous carbon was irregular particle.
Herein, we report a facile route to produce high special surface area, large pore volume of nanosheet nitrogen-containing porous carbon from cellulose. When used for CO2 adsorption, the adsorption capacity is reached up to 5.8 mmol g−1 at room temperature due to its unique compositional and structural features; it is much higher than reported porous carbon in literatures.
Experimental section
Synthesis of porous carbon nanosheets
Cellulose was selected as the raw materials for preparing nanosheet porous carbon. In a typical synthesis, after mixing cellulose with mixture solution of KOH and urea (H2O–KOH–urea–cellulose = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 wt%), the mixture precursor was dried at 80 °C, then, it was carbonized in a tube furnace at 600 °C or 800 °C for 1 h under nitrogen flow. The obtained sample was subsequently washed in distilled water to remove any metal residue. The samples were noted as C-KU-600 and C-KU-800 according to the carbonization temperature. The pristine cellulose and cellulose impregnated with KOH were also carbonized at 800 °C and noted as C-O-800 and C-K-800.
12 wt%), the mixture precursor was dried at 80 °C, then, it was carbonized in a tube furnace at 600 °C or 800 °C for 1 h under nitrogen flow. The obtained sample was subsequently washed in distilled water to remove any metal residue. The samples were noted as C-KU-600 and C-KU-800 according to the carbonization temperature. The pristine cellulose and cellulose impregnated with KOH were also carbonized at 800 °C and noted as C-O-800 and C-K-800.
Structural characterization
Nitrogen adsorption–desorption isotherms were measured at −196 °C on a Micromeritics ASAP 2020 adsorption apparatus. Before the measurements, all the samples were degassed at 180 °C for more than 6 h under high vacuum conditions. The surface area was calculated by the Brunauer–Emmett–Teller (BET) method from the adsorption branch in the relative pressure range (P/P0) of 0.01 to 0.2. The pore size distribution (PSD) was calculated by a non-local density functional theory (NLDFT) method using nitrogen adsorption data and assuming a slit pore model. The total pore volumes (Vtotal) were estimated in terms of the adsorption amount at P/P0 of 0.99. The SEM images were obtained on a S-4800 Field Emission-Scanning Electron Microscope (Hitachi, Japan) operated at 1 kV. Transmission electron microscopy (TEM) was carried out with a JEM-2000 FX instrument operating at a 200 kV accelerating voltage. Fourier transform infrared (FT-IR) spectra of the samples were measured on a Nicolet FT-IR 380 spectrometer using the conventional KBr pellet method. Thermal gravimetric analysis (TGA) was performed under argon at 30–800 °C with a heating rate of 10 °C min by a thermogravimetric analyzer (Rigaku, TG-DTA 8120, Japan), and the exhaust gases were detected by subsequent mass spectroscopy (Omni Star). The X-ray photoelectron spectroscopy (XPS) was measured with an ESCALAB 250 (Thermo Electron). The X-ray excitation was provided by a monochromatic Al Kα (1486.6 eV) source. Survey scans were obtained using a pass energy of 100 eV while high resolution scans of specific elements were obtained using a 20 eV pass energy. The detection of the emitted photoelectrons was performed perpendicular to the surface sample. Data quantification was performed on the advantage program.CO2-capture measurements
The CO2-adsorption isotherms of the porous carbons were measured using a Micromeritics ASAP 2020 static volumetric analyzer at 25 °C. Prior to each adsorption experiment, the sample was degassed for 2 h at 150 °C ensuring that the residual pressure fell below 5 × 10−3 mbar and then cooled down to 25 °C, followed by the introduction of CO2 into the system. The CO2-adsorption capacity in terms of adsorbed volume under standard temperature and pressure (STP) was then recorded.Results and discussion
KOH activation has been widely used on grapheme,22 carbon nanotubes,23 carbide-derived carbon, and mesoporous carbon24 to increase the surface area and improve the electrochemical performance. When the activation temperature is higher than 400 °C, The chemical reactions are involved into the process: 6KOH + 2C → 2K + 3H2 + 2K2CO3, and subsequent decomposition of K2CO3 and/or reactions of K/K2CO3/CO2 with the carbon.25 Given the loss of carbon, a large amount of nanoscale pores are generated in the final products. Scheme 1 outlines the strategy used to synthesize the nanosheet microporous carbon from cellulose. Firstly, cellulose was dispersed into the solution of KOH and urea, the water was evaporated at 80 °C, the aggregated KOH and urea could further crystallize to form layer structure, and the cellulose coated on the layer surface. Then, the composites were subsequently chemically activated with KOH at 600 °C or 800 °C for 1 h under a nitrogen atmosphere, during heating and activation process, the cellulose was performed polymerization and pyrolysis reactions, the urea was decomposed and reacted with carbon and KOH, meanwhile the KOH was reacted with carbon, resulting in porous nitrogen-doped carbon nanosheets with large surface areas. By this pyrolysis, condensation, polymerization, carbonization and activation process, the nitrogen atoms from urea are expected to replace the carbon atoms in the carbon matrix sheets. As illustrated in Scheme 1, in this manner, we hope to make use of the better hydrophily of heteroatom-doped carbon materials as well as the open, shortened paths offered by nanosheet microporous carbon materials.The TGA curves and pyrolysized gases of urea, pristine cellulose, cellulose with urea, cellulose with KOH, and cellulose with KOH–urea showed weight loss in different stages in Fig. 1. The pyrolysized gases of CO, CO2, NH3 and HCN were produced at different temperatures were listed in Table 1 and the relative intensities vs. temperature were shown in Fig. 1S.† For pristine cellulose, the first stage of weight loss is about 10% in between 50 °C and 200 °C. This may be due to the loss of adsorbed and bound water. The second stage ranged from 200 °C to 350 °C with the fastest decomposition rate, 50% weight loss happens due to degradation of cellulose at 350 °C. A slower weight loss appears from 350 °C to 700 °C due to the pyrolysis and decomposition of cellulose to form condensation cross-linking ring structure. For the cellulose with urea, the degradation behavior is different from cellulose. Here, the first stage of weight loss is due to the removal of water content lower at 150 °C. The onset degradation temperature starts earlier at 150 °C due to urea decomposing and a fastest weight loss rate occurs from 230 °C to 320 °C because of cotton pyrolysis with urea decomposition. When cellulose impregnated with KOH, the weight was gradually decreased from 200 °C to 500 °C. The weight loss behavior of cotton impregnated with KOH and urea showed two fast weight loss rates before 300 °C, the presence of KOH, which promote the decomposition of urea. The carbon yields based on cellulose of pristine cellulose, cellulose with urea, cellulose with KOH, and cellulose with KOH–urea were 13%, 17%, 23% and 25%, respectively. When cellulose impregnated with urea or KOH–urea, the HCN was recorded at higher temperature than that from urea, which indicated that the product of urea might connected with cellulose and was further decomposed to HCN at higher temperature. Based on the exhausted gases forming process, the nitrogen element might be incorporated into carbon matrix via nitrogen radius from urea decomposition during heat treatment.
| Samples | Temperature (°C) | Exhausted gases relative content (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CO | CO2 | NH3 | HCN | CO | CO2 | NH3 | HCN | |
| Urea | 150–300 | 170–240; 280–350; 360–650 | 150–300 | 300–370 | 16.03 | 53.44 | 29.75 | 0.78 |
| Cellulose | 250–350 | 250–350 | — | — | 48.95 | 49.94 | — | — |
| Cellulose–urea | 150–370 | 150–225; 250–400 | 150–250; 350–500 | 300–550; 550–800 | 30.82 | 36.69 | 28.99 | 3.49 |
| Cellulose–KOH | 200–350; 350–450; 600–800 | 170–270; 410–470; 550–800 | — | — | 61.93 | 37.46 | — | — |
| Cellulose–KOH–urea | 170–280; 370–450; 600–800 | 150–250; 250–400; 500–750 | 170–250; 250–400 | 250-570; 650–800 | 40.54 | 36.14 | 22.09 | 0.93 |
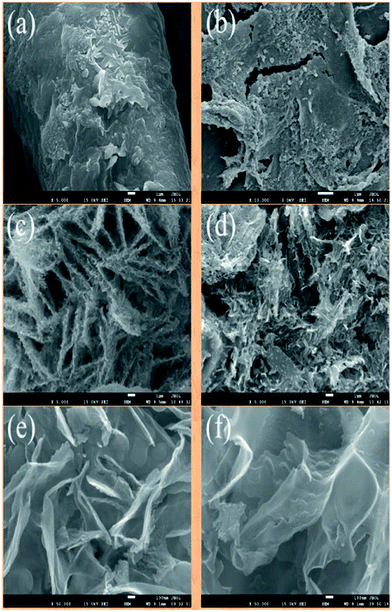
The morphologies of the products at different stages were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) and were shown in Fig. 2 and 3. The resulted nanosheet porous carbons obviously maintained their sheet structure, and the thick is about 10 nm (Fig. 2(e) and (f)). The morphologies of activation products were dependent on the temperature, it is regular piling up after 600 °C activation; but it is irregular assembling after 800 °C activation. When the potassium salt was removed, nanosheet porous carbon with 10–20 nm could be obtained. Compared with cellulose impregnated with KOH or urea, the resulted porous carbons displayed aggregative and irregular particles (see Fig. S2 and S3†). This result suggests that, as expected, most of the cellulose are concentrated on the surface of KOH and urea during drying process, thus the morphology of residual carbon are kept after activation. The HRTEM image in Fig. 3(b) and (d) indicate that nanosheet porous carbon of C-KU-600 and C-KU-800 mainly contained micropore less than 2 nm, classical structure of biomass carbon. Fig. 4S† displayed different magnification of C-KU-600 and C-KU-800. Meanwhile, the nanosheet was twisted of C-KU-800; this was possible of serious reaction between KOH and carbon at high temperature.
 | ||
| Fig. 3 HRTEM images of nanosheet porous carbons at different resolutions. (a and b) C-KU-600; (C) and (D) C-KU-800. | ||
The specific surface areas and pore size distributions of samples were measured by N2 adsorption–desorption isotherms (Fig. 4(a)). The pore structure parameters of samples were listed in Table 2. The nanosheet carbons exhibited type I isotherms, and a high Brunauer–Emmett–Teller surface area of 1854 m2 g−1 for C-KU-800 and 1138 m2 g−1 for C-KU-600. The pore size distribution curves derived from the adsorption branch of isotherms using nonlocal density functional theory (NLDFT) method are presented in Fig. 4(b). The C-KU-800 with a high pore volume of 0.82 cm3 g−1 mainly possesses micropores (peak at 0.74, 1.10 and 1.74 nm); and showed stronger pore size distributions intensities in 0.9–1.1 nm and 1.7–3 nm than that of C-KU-600, this is again corroborated the HRTEM results. Compared with C-K-800 (surface area of 1436 m2 g−1 and pore volume of 0.697 cm3 g−1), the higher surface area and pore volume of C-KU-800 may be resulted from the dispersion of cellulose with KOH–urea, and the resultant porous carbon morphology. Although porous carbon from pristine cellulose displayed type I isotherm, it showed lower surface area (460 m2 g−1) and less pore volume (0.22 cm3 g−1), this indicated that the cellulose impregnated with KOH and urea, could evolve nanosheet structure and promote the pore structure developing.
 | ||
| Fig. 4 (a) N2 sorption isotherms and (b) NLDFT pore size distributions of samples (C-O-800, C-K-800, C-KU-600 and C-KU-800). | ||
| Samples | Elemental compositiona (wt%) | Structure parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | SBETb m2 g−1 | SMicroc m2 g−1 | Vtotald cm3 g−1 | VMicro cm3 g−1 | |
| a Data from elemental analysis.b Specific surface area from multiple BET method.c Micropore surface area from t-plot method.d Total pore volume at (P/P0 = 0.99). | ||||||||
| C-800 | 92.73 | 0.552 | — | 6.72 | 460 | 419 | 0.22 | 0.18 |
| C-K-800 | 91.94 | 0.45 | — | 7.61 | 1436 | 1238 | 0.697 | 0.446 |
| C-KU-600 | 83.18 | 0.82 | 3.57 | 12.43 | 1138 | 894 | 0.55 | 0.45 |
| C-KU-800 | 88.75 | 0.80 | 1.37 | 9.08 | 1854 | 1351 | 0.82 | 0.53 |
The Fourier transformed infrared (FT-IR) spectra of pristine cellulose, C-O-800, C-KU-600 and C-KU-800 were shown in Fig. S5,† in which the main absorption peaks of functional groups, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, C–H, and –O–H, gradually became weak and finally disappeared with carbonization temperature, thereby indicating the carbonization temperature increase promotes the surface hydrophobic.
O, C–O, C–H, and –O–H, gradually became weak and finally disappeared with carbonization temperature, thereby indicating the carbonization temperature increase promotes the surface hydrophobic.
To confirm the chemical composition, X-ray photoelectron spectroscopy (XPS) and elemental analysis were conducted. As shown in Fig. 5 and S6,† three typical peaks corresponding to the binding energies of C 1s, N 1s, and O 1s are observed in the wide XPS survey scan for C-KU-600 and C-KU-800. In contrast to C-KU-600, the N1s peak is weak in C-KU-800. Three types of nitrogen configurations and related contents: pyridine-like (N-6), pyrrole-like (N-5), and graphite-like (N-Q) were illustrated in C-KU-600 and C-KU-800 (Fig. 6 and Table S1†). The three nitrogen configurations related peaks area ratio for C-KU-600 and C-KU-800 were 1.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.82
1.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.27
1 and 2.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.60
2.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Quantitative analysis elucidates the corresponding atom contents (Table 2) were consistent with that derived from elemental analysis. Moreover, the contents of pyridine-like (N-6) and pyrrole-like (N-5) increased, but the graphite-like (N-Q) decreased with activation temperature. This discrepancy is in accordance with the previous observation that the nitrogen functional groups are decreased with activation temperature.26
1, respectively. Quantitative analysis elucidates the corresponding atom contents (Table 2) were consistent with that derived from elemental analysis. Moreover, the contents of pyridine-like (N-6) and pyrrole-like (N-5) increased, but the graphite-like (N-Q) decreased with activation temperature. This discrepancy is in accordance with the previous observation that the nitrogen functional groups are decreased with activation temperature.26
 | ||
| Fig. 6 (a) CO2 adsorption isotherms of C-O-800, C-K-800, C-KU-600 and C-KU-800; (b) CO2-TPD curves of C-K-800, C-KU-600 and C-KU-800. | ||
The C 1s XPS spectra obtained for C-KU-600 and C-KU-800, showed rather complex deconvoluted patterns (Fig. S6†). Both C-KU-600 and C-KU-800 exhibited a broad peak at 284.6 eV and this peak could be deconvoluted into several distinct peaks at 284.6, 285.7 and 288.2 eV for C-KU-600 and 284.3, 284.6, 285.6, 286.6, and 288.3 eV for C-KU-800, this revealed the presence of signals from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV), C–O or C–N (285.7 eV), C
C (284.6 eV), C–O or C–N (285.7 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C
O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (288.2 eV). In addition, the O 1s peaks of C-KU-600 and C-KU-800 (Fig. S6†), which can be ascribed to adsorbed oxygen (531.2 eV),
N (288.2 eV). In addition, the O 1s peaks of C-KU-600 and C-KU-800 (Fig. S6†), which can be ascribed to adsorbed oxygen (531.2 eV), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/–C–O–C–(532.2 eV), –O–C
O/–C–O–C–(532.2 eV), –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (533.2 eV), respectively.27
O (533.2 eV), respectively.27
The above characterizations proved the existence of oxygen and nitrogen-containing groups in the nanosheet microporous carbons prepared from cellulose. Its adsorption isotherms of CO2 were drawn in Fig. 6(a), the CO2 uptakes by C–K-800, C-KU-600 and C-KU-800 were 4.1 mmol g−1, 5.0 mmol g−1 and 5.8 mmol g−1, respectively. Compared with the reported cellulose-based high surface area porous carbon (SBET = 2250 m2 g−1, Vtotal = 1.01 cm3 g−1),28 the nanosheet porous carbon had less surface area and pore volume, but its CO2 adsorption capacity was same to that of high surface area porous carbon (5.8 mmol g−1), this indicated that the nitrogen doping nanosheet porous carbon was potential materials for CO2 capture due to its unique compositional and structural features. Moreover, the adsorption capacity of CO2 was 2.0 mmol g−1 at 0.15 bar, it is interesting for flue gas CO2 capture. Fig. 6(b) shows the CO2-TPD profiles of C-KU-600 C-KU-800, there are three desorption peaks appearing before 150 °C, 150–230 °C and 230–300 °C, which represent the physical adsorbed CO2, poor and strong chemical adsorbed CO2. The TPD-CO2 of C-K-800 illustrated that there was mainly physical adsorption due to no nitrogen-containing groups on its surface. This suggested that large surface area, pore volume and surface rich of basic sites are necessary for achieving high CO2 adsorption capacity.
Physical adsorption for CO2 was mainly taken place at micropore range of porous carbon,29 C-KU-800 showed higher surface area and pore volume than that of C-KU-600, but the difference of their microporous volume is less (0.53 cm3 g−1 vs. 0.45 cm3 g−1). The nitrogen content of C-KU-600 is 2.6 times of C-KU-800 (3.57 wt% vs. 1.37 wt%), and it has more N-Q species, so, a higher CO2 desorption peak of C-KU-600 appears between 250 °C and 300 °C. In addition, the nitrogen groups in external surface and large pore wall of C-KU-600 and C-KU-800 may adsorb CO2. Compared with C-KU-800 and C-K-800, C-KU-600 with less surface area and microporous volume, but it still displayed better adsorption property for CO2 due to its more nitrogen groups. Summarized above factors, CO2 adsorption was determined by the microporous structure and surface nitrogen groups.
In order to compare the effect of NaOH and KOH on the pore structure and morphology of cellulose-based porous carbon, the cellulose was impregnated with NaOH and urea (same molar ratio as KOH), and was activated at 800 °C. The nitrogen adsorption isotherm, CO2 adsorption isotherm and morphology of resultant porous carbon (C-NaU-800) were showed in Fig. S7–S9.† The special surface area and pore volume of porous carbon from cellulose impregnated with NaOH/urea are less than that of C-KU-800; its pore size distribution is wider than that of C-KU-800. In general, KOH produces porous carbons with narrower micropore distributions than those prepared by NaOH.30 The C-NaU-800 is irregular particles; this may be determined by the chemical property of NaOH. The CO2 adsorption capacity on C-NaU-800 reached to 4.5 mmol g−1, but the adsorption isotherm was gentler than that of C-KU-800 and C-KU-600, this suggested that the pore structure and morphology determined the CO2 adsorption behavior on porous carbons. The nanosheet structure minimizes CO2 diffusion limitations and allows rapid adsorption.
Conclusions
Nitrogen-doped porous carbon sheets are successfully fabricated via chemical activation of cellulose impregnated with KOH and urea. Unique compositional and structural features endow the nitrogen-doped porous carbon nanosheets with superior CO2 adsorption performance of 5.8 mmol g−1.Notes and references
- (a) S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169 CrossRef CAS PubMed; (b) C. D. Liang, Z. J. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696 CrossRef CAS PubMed; (c) Y. Wan, Y. F. Shi and D. Y. Zhao, Chem. Mater., 2008, 20, 932 CrossRef CAS.
- (a) X. Wang, J. C. Yu, Y. Hou and X. Fu, Adv. Mater., 2005, 17, 99 CrossRef CAS; (b) S. E. Skrabalak and K. S. Suslick, J. Am. Chem. Soc., 2005, 127, 9990 CrossRef CAS PubMed.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87 CrossRef CAS PubMed.
- K. J. Lee, N. Shiratori, G. H. Lee, J. Miyawaki, I. Mochida, S. H. Yoon and J. Jang, Carbon, 2010, 48, 4248 CrossRef CAS.
- D. H. Deng, L. Yu, X. Q. Chen, G. X. Wang, L. Jin, X. L. Pan, J. Deng, G. Q. Sun and X. H. Bao, Angew. Chem., Int. Ed., 2013, 52, 371 CrossRef CAS PubMed.
- (a) X. H. Zhong, Y. L. Li and J. M. Feng, Nanoscale, 2012, 4, 5614 RSC; (b) Z. Shi, X. J. Chen, X. W. Wang, T. Zhang and J. Jin, Adv. Funct. Mater., 2011, 21, 4358 CrossRef CAS.
- M. Oschatz, E. Kockrick, M. Rose, L. Borchardt, N. Klein, I. Senkovska, T. Freudenberg, Y. Korenblit, G. Yushin and S. Kaskel, Carbon, 2010, 48, 3987 CrossRef CAS.
- F. J. Li, M. Morris and K.-Y. Chan, J. Mater. Chem., 2011, 21, 8880 RSC.
- Y. B. Jiang, L. H. Wei, Y. Z. Yu and T. Zhao, eXPRESS Polym. Lett., 2007, 1, 292 CrossRef CAS.
- Y. Fang, Y. G. Lv, R. C. Che, H. Y. Wu, X. H. Zhang, D. Gu, G. F. Zheng and D. Zhao, J. Am. Chem. Soc., 2013, 135, 1524 CrossRef CAS PubMed.
- (a) H. G. Wang, Z. Wu, F. L. Meng, D. L. Ma, X. L. Huang, L. M. Wang and X.-B. Zhang, ChemSusChem, 2013, 6, 56 CrossRef PubMed; (b) F. Su, C. K. Poh, J. S. Chen, G. Xu, D. Wang, Q. Li, J. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717 RSC.
- (a) W. Z. Shen, S. C. Zhang, Y. He, J. F. Li and W. B. Fan, J. Mater. Chem., 2011, 21, 14036 RSC; (b) R. Ansari and N. K. Fahim, React. Funct. Polym., 2007, 67, 367 CrossRef CAS.
- (a) Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362 CrossRef CAS PubMed; (b) K. Chizari, A. Deneuve, O. Ersen, I. Florea, Y. Liu, D. Edouard, I. Janowska, D. Begin and C. Pham-Huu, ChemSusChem, 2012, 5, 102 CrossRef CAS PubMed.
- N. Fechler, T. P. Fellinger and M. Antonietti, Adv. Mater., 2013, 25, 75 CrossRef CAS PubMed.
- K. K. R. Datta, D. Jagadeesan, C. Kulkarni, A. Kamath, R. Datta and M. Eswaramoorthy, Angew. Chem., Int. Ed., 2011, 50, 3929 CrossRef CAS PubMed.
- Z. Y. Wu, C. Li, H. W. Liang, J. F. Chen and S. H. Yu, Angew. Chem., Int. Ed., 2013, 52, 2925 CrossRef CAS PubMed.
- M. Mecklenburg, A. Schuchardt, Y. K. Mishra, S. Kaps, R. Adelung, A. Lotnyk, L. Kienle and K. Schulte, Adv. Mater., 2012, 24, 3486 CrossRef CAS PubMed.
- Y. S. Yun, S. Y. Cho, J. Shim, B. H. Kim, S. J. Chang, S. J. Baek, Y. S. Huh, Y. Tak, Y. W. Park, S. Park and H. J. Jin, Adv. Mater., 2013, 25, 1993 CrossRef CAS PubMed.
- P. Niu, L. L. Zhang, G. Liu and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 4763 CrossRef CAS.
- (a) T. Sasaki, M. Watanabe, H. Hashizume, H. Yamada and H. Nakazawa, J. Am. Chem. Soc., 1996, 118, 8329 CrossRef CAS; (b) Y. Omomo, T. Sasaki, L. Z. Wang and M. Watanabe, J. Am. Chem. Soc., 2003, 125, 3568 CrossRef CAS PubMed; (c) S. Ithurria, M. D. Tessier, B. Mahler, R. P. S. M. Lobo, B. Dubertret and A. L. Efros, Nat. Mater., 2011, 10, 936 CrossRef CAS PubMed; (d) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- (a) Y. S. Yun, J. Shim, Y. Tak and H. J. Jin, RSC Adv., 2012, 2, 4353–4358 RSC; (b) Y. S. Yun, D. Kim, H. H. Park, Y. Tak and H. J. Jin, Synth. Met., 2012, 162, 2337–2341 CrossRef CAS; (c) Y. S. Yun and H. J. Jin, Mater. Lett., 2013, 108, 311–315 CrossRef CAS.
- Y. W. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537 CrossRef CAS PubMed.
- E. Raymundo-Piñero, P. Azaïs, T. Cacciaguerra, D. Cazorla-Amorös, A. Linares-Solano and F. Béguin, Carbon, 2005, 43, 786 CrossRef.
- J. Jin, S. Tanaka, Y. Egashira and N. Nishiyama, Carbon, 2010, 48, 1985 CrossRef CAS.
- M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2003, 41, 267 CrossRef.
- (a) M. Molina-Sabio and F. Rodríguez-Reinoso, Colloids Surf., A, 2004, 24, 15 CrossRef; (b) L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202 CrossRef CAS PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobel, K. D. Bomben, Handbook of X-Ray Photoelectron Spectroscopy – A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, Perkin-Elmer Corporation, Eden Prairie, MN, USA 1992 Search PubMed.
- M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 1765 CAS.
- H. R. Wei, S. B. Deng, B. Y. Hu, Z. H. Chen, B. Wang, J. Huang and G. Yu, ChemSusChem, 2012, 5, 2354 CrossRef CAS PubMed.
- P. J. M. Carrott, M. M. L. Ribeiro Carrott and Suhas, Carbon, 2010, 48, 4162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Exhaust gases compositions, FT-IR spectra, SEM images of the cellulose with urea or KOH, TEM images of nanosheet porous carbons, C1s and O1s XPS spectra, and different nitrogen group contents. See DOI: 10.1039/c3ra47946c |
| This journal is © The Royal Society of Chemistry 2014 |